Retroviral Integrase
From Proteopedia
|
Structural basis of retroviral DNA integration & Modeling in the development of antiretroviral drugs
New Article
|
| About • Editing • Help | Video Guide • Table of Contents • Content (Topic Pages) • What's New |
Contents |
Introduction
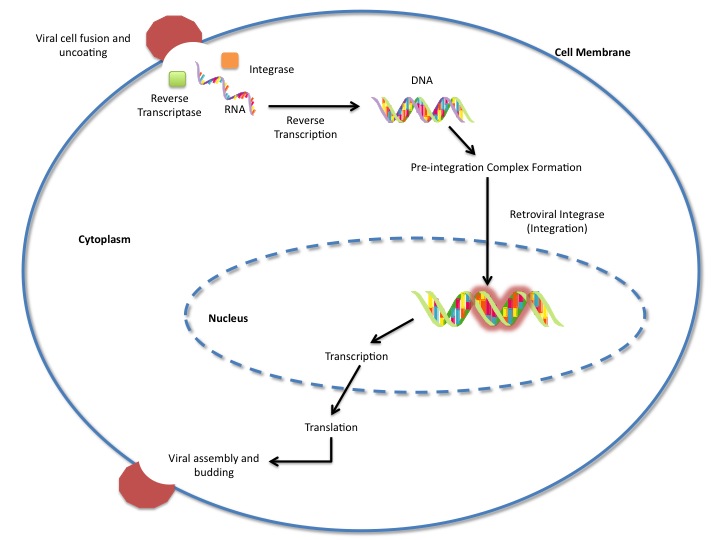
Integrase is an essential retroviral enzyme that binds to viral DNA and inserts it into a host cell chromosome. [The reverse transcribed cDNA of human immunodeficiency virus type 1 (HIV-1) is inserted in the host cell genome in order increase pathogen fitness and virulence.]It is produced by a class of retrovirus (like HIV) and is used by the virus to incorporate its genetic material into the host cell DNA. The host cellular machinery then produces mRNA and then protein from the incorporated genetic material, thus replicating the virus. Although several integrase inhibiting drugs have been investigated, the mechanism [responsible for strand-transfer inhibition action remain unclear] of action was not clear. On February 1, 2010, a crystal structure of the PFV Intasome was published, paving the way for better retroviral treatments. [However, Hare et al (2010) determined the structural constituents of retroviral integration. Further elucidation of the complete structure of the retroviral integrase, and its application to regulate functional and enzymatic activities could potentially enable researchers to delay the progression of retroviral diseases. Moreover, study of HIV-1 integration could lead to a promising new target, and contribute to the generation pharmacophore models for antiviral therapy. ]
HIV Integrase inhibitors: Raltegravir, marketed as Isentress is currently approved as a therapeutic inhibitor of HIV integrase. It was approved on October 12, 2007. [link below has a table of antiretroviral drugs with trade name, company, patents, and notes]
Integrase Mechanism of Action
HIV and AIDS
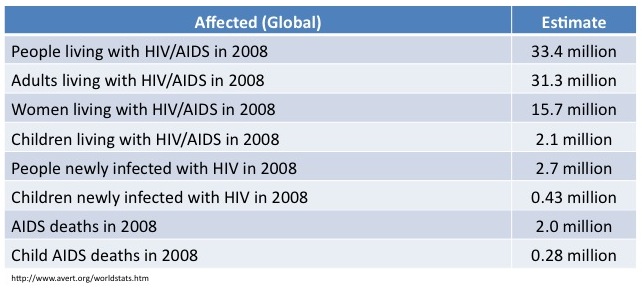
To date, more than 25 million people have died of AIDS and it is estimated that approximately 33 million people are living with HIV today.
Impact of Structure
HIV protease and integrase structures are among the highest ranked structures that have contributed to saving many lives and added to the quality of life of many HIV-afflicted individuals. It is implemented in structure-based drug design to develop protease inhibitors and integrase inhibitors, and is used as a significant component of highly active anti-retroviral therapy (HAART).
While existing antiretroviral agents improve the quality of life as well as extending the life of many patients, it fails to eradicate the disease. Studies in integrase inhibitors show that combination with other antiretroviral drugs diminish viral adaptations, and may have the potential to be used for salvage therapy for patients who have acquired resistance to other drugs. For more, please see AIDS Before Protease Inhibitors & HIV Protease Inhibitors: A Breakthrough.
PFV Intasome Crystallization
To mimic the viral DNA ends of HIV-1, Hare et al (2010) utilized soluble and fully functional prototype foamy virus (PFV) intasome preparations, obtained using recombinant PFV integrase and double-stranded oligonucleotides.
The remarkable stability of the integrase-DNA complexes were determined by observing the in vitro strand transfer reactions, which were classified into three modes of deproteination migration: (1) single concerted events: linearized target plasmid; (2) multiple concerted events: smear; (3) half-site events: open circular DNA. Further characterization of the PFV intasome also exhibited structural substantiality which implied strong protein-protein and protein-DNA interactions despite prolonged incubation under high ionic strength conditions. Comprehensive crystallization assays effected a viable crystal configuration that diffracted X-rays to 2.9 Angstroms resolution. A three-dimensional structure was ultimately determined. The asymmetric unit contained a single integrase dimer with a stably bound viral DNA molecule, and a pair of integrase dimers consociated with symmetry, which formed an oblong tetramer. The dimer interface is stabilized by intermolecular amino terminal and catalytic core domains (inner subunit-outer subunit) interactions. The overall shape of the oblong tetramer is unique albeit bearing semblances to previously reported HIV-1 integrase complexes.
Overall Architecture & Components
Active Site
Mechanism for strand-transfer inhibition action
HIV integrase type 1 is a relatively new and novel target for inhibitors. In 2007, the first HIV-1 integrase inhibitor, Raltegravir, was approved by the FDA (6). Strand-transfer inhibitors work by preventing the concerted integration of the viral DNA into the host chromosome. After viral entry into the host cell, the reverse transcriptase converts the viral ssRNA into dsDNA. At this point, the integrase forms a complex with the viral DNA, creating the pre-integration complex (intasome). The pre-integration complex is then chaperoned into the nucleus, where two nucleotides are excised from the 3' end. Next, the DNA is then covalently integrated into the host DNA. The strand=transfer inhibitors interrupt this process, preventing the integration of the viral DNA into the host chromosome.
Integrase Inhibitors
| Name | Brand | Company | Patent | Notes |
| Raltegravir | Isentress | Merck & Co. | - | also known as MK-0518. The isopropyl and methyl-oxadiazole of MK-0518 are involved in hydrophobic and stacking interactions with side chains of Pro 214 and Tyr 212 to stabilize this drug within the PFV intasome active site. This manner of drug-binding interaction causes displacement of the reactive 3' viral DNA end from the active site of PFV intasome. After binding of MK-0518 to active site, the reactive 3' hydroxyl group moves away from the active site of the PFV intasome by more than 6 Angstroms. Raltegravir was approved by the FDA on October 12, 2007, for use with other anti-HIV agents in the treatment of HIV infection in adults. It is the first integrase inhibitor approved by the FDA. |
| Elvitegravir | - | Gilead Science | - | GS-9137 interacts with Pro 214 of PFV intasome through its quinolone base and isopropyl group. In experimental stages; shares the core structure of quinolone antibiotics. Phase II studies of elvitegravir in people who are treatment experienced have been completed. Phase III studies in treatment experienced patients are ongoing. A phase II study of elvitegravir in people who have never taken antiretroviral therapy is underway. This study will also be evaluated a boosting agent in place of Norvir, currently called GS9350. Elvitegravir holds promise for HIV-positive patients who have taken other anti-HIV drugs in the past. |
| MK-2048 | - | Merck & Co. | - | A second generation integrase inhibitor, intended to be used against HIV infection. It is superior to the first available integrase inhibitor, raltegravir, in that it inhibits the HIV enzyme integrase 4 times longer. It is being investigated for use as part of pre-exposure prophylaxis (PrEP). |
References
1.Hare, Stephen; Gupta, Saumya Shree; Valkov, Eugene; Engelman, Alan & Cherepanov, Peter (2010) Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 2010/01/31/online doi:10.1038/nature08784 <http://www.nature.com/nature/journal/vaop/ncurrent/full/nature08784.html> Template:STRUCTURE 3l2q
2. http://www.isentress.com/raltegravir/isentress/consumer/index.jsp
3. deJesus, Edwin HIV Antiretroviral Agents in Development. The Body: The Complete HIV/AIDS Resource. March 30, 2006.
4. AIDS Info
5. Krishan K. Pandey and Duane P. Grandgenett (2008) HIV-1 Integrase Strand Transfer Inhibitors: Novel Insights into their Mechanism of Action. Retrovirology: Research and Treatment" 2008:2 11-16
6.James F. Braun, DO, Ruth J. Cronje, PhD, Marnie G. Henderson (2008) HIV-1 Integrase Inhibitors. www.prn.org Volume 13, Pages 1–9
Further Reading
- GEN News Highlights "Scientists Solve 3-D Crystal Structure of Retroviral Integrase Bound to Viral DNA", Genetic Engineering & Biotechnology News February 1, 2010.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Rhysly Martinez, Joel L. Sussman, Alexander Berchansky, David Canner, Jordan Heard, Eugene Babcock, Garrett Asanuma