Sandbox 181
From Proteopedia
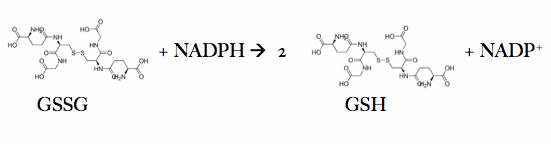
Glutathione reductase, also known as GSH reductase, is found in the human erythrocytes and converts oxidized glutathione (GSSG) to two molecules of reduced gluthatione (GSH).
| |||||||
| 3djj, resolution 1.10Å () | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , , , , | ||||||
| Gene: | GSR, GLUR, GRD1 (HUMAN) | ||||||
| Activity: | Glutathione-disulfide reductase, with EC number 1.8.1.7 | ||||||
| Related: | 3djg | ||||||
| |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Contents |
Structure
Glutathione reductase belongs to the larger family of flavoezymes, which use a flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) in catalysis. It is an oxiodreductase homodimer of 52kD monomers of which, each has three domains: () 1. NADPH-binding domain (yellow), 2. FAD-binding domain (red), 3. dimerization domain.
Reaction
The action of glutathione reductase proceeds through a cyclic series of structures in differing redox states . NADPH binds causing a transient reduction of flavin and this reduced flavin consequently reduces Cys58-Cys63 disulfide bond, forming a short lived covalent intermediate with Cys63. Following this, a stable charge-transfer complex between flavin and the Cys63 thiolate forms. After formation the NADP+ dissociates and is replaced by another NADPH. This is the end of the reductive first half of the mechanism and the oxidative half is initiated upon the binding of GSSG. The Cys58 in glutathione reductase attacks CysI of the GSSG to form a mixed disulfide between the first GS and Cys58. The second GSH is the free to leave and the disulfide bond is reformed between Cys58 and Cys63 of glutathione reductase. Finally, the first molecule of GSH is released. The glutathione reductase is then able to be recycled to allow for the binding of NADPH once again[1].
Mechanism
Function
For proper functioning and prevention of damage to a cell, glutathione plays an essential role in preventing oxidative stress. For example, GSH can directly scavenge hydroxyl radicals and singlet oxygens and can regenerate important antioxidants such as Vitamins E and C to their reactive forms [2]. The antioxidant capacity of glutathione is linked to the redox state of GSSG/2GSH inside the cell[3]. The function of glutathione reductase is to maintain this narrow redox state of high reduced to oxidized GSH in the cell[4].
Consequences of a Mutation
References
- ↑ Berkholz DS, Faber HR, Savvides SN, Karplus PA. Catalytic cycle of human glutathione reductase near 1 A resolution. J Mol Biol. 2008 Oct 3;382(2):371-84. Epub 2008 Jul 7. PMID:18638483 doi:10.1016/j.jmb.2008.06.083
- ↑ Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005 Oct;16(10):577-86. PMID:16111877 doi:10.1016/j.jnutbio.2005.05.013
- ↑ Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003 Jul 1;333(1):19-39. PMID:12809732
- ↑ Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005 Oct;16(10):577-86. PMID:16111877 doi:10.1016/j.jnutbio.2005.05.013
Human Glutathione Reductase
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |