Sandbox 177
From Proteopedia
| |||||||||
| 3es9, resolution 3.40Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Gene: | CYPOR, Por (Rattus norvegicus) | ||||||||
| Activity: | NADPH--hemoprotein reductase, with EC number 1.6.2.4 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
NADPH-cytochrome P450 oxidoreductase
Taya O'Neill
General Information
Horecker first identified this protein in 1950 as NADPH-specific cytochrome c reductase, based on his assumption that it was the redox partner for cytochrome c, found in the mitochondria.[1] However, studies in the 1960s and later showed that its main function is actually as the redox partner for cytochrome P450 in microsomal electron transport chains, resulting in a name change.[2][3] Today this protein is known as NADPH-cytochrome P450 oxidoreductase (CYPOR).
CYPOR is a ~78kDa, multidomain flavoprotein.[2] Containing three co-factors, FAD, FMN and NADPH, this protein is the archetype for the mammalian diflavin-containing enzyme family.[2] Research indicates that the protein, and other FAD/FMN binding proteins, are likely the product of the fusion of two ancestral genes.[4] This would account for the two distinct binding domain areas, FMN and FAD/NADPH, which each provide different functional capabilities to the overall protein.[4]
Regulation of this protein, which is found all tissues to some extent, is largely at the transcriptional level.[5] The thyroid hormone T3 acts as a hormonal regulator in most cases, while adrenocorticotrophic hormone acts as a regulator in a few specific cases.[6][7]
Structure
|
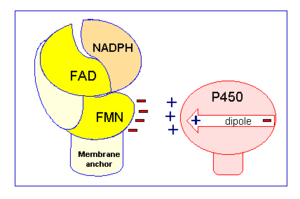
CYPOR is a complex, multidomain protein composed of three chains (, , ). It also has three different types of associated ligands; one , three and two (Fig 1).[2] The three associated binding domains for these ligand types, a connecting domain and a transmembrane anchor make up the important structural elements of CYPOR (Fig 2).
The N-terminus of CYPOR consists of a single alpha-helix that functions as a transmembrane anchor (~6kDa), holding the protein in the endoplasmic reticulum.[2] The remaining, soluble ~66kDa portion of the protein is responsible for reducing cytochrome P450, consisting of three binding domains for the ligands responsible for the electron flow.[2] The FMN binding domain is composed of the first 170 residues of the soluble region, which are very similar to those of flavodoxin, another FMN binding protein.[2] The FAD and NADPH binding domains are located closer to the C-terminus, and are very similar to the FAD domain in ferredoxin-NADP+ oxidoreductase, both in terms of sequence and structure.[2]
Between the FMN and FAD/NADPH bind domains is a connecting domain, which is a highly flexible random coil.[2] The hinge region is composed of 12 residues from Gly232 to Arg243, and is highly conserved among most known CYPOR proteins, including those found in humans, rats and even yeast.[2] This section is presumed to be responsible for the relatively increased mobility of the FMN domain, changes to conformation and the relative orientation of the binding domains.[2] Studies that examined the rate of electron transfer within CYPOR seem to confirm this, as electron transfer rate appears to decrease proportionally to increases in the viscosity of the fluid medium it is in.[2] When residues within the the hinge are mutated so it is no longer flexible, studies have found that CYPOR cannot effectively transfer electrons to cytochrome P450, unless there is a high electron pool available.[2] This indicates that without the hinge movement electrons are not able to be efficiently moved from FAD to FMN, decreasing the reductase capabilities of CYPOR.[2] These surface proteins are
Function
In vivo CYPOR is believe to alternate between a one and a three electron reduced form. While the 1 electron form is fairly stable, forming a neutral blue semiquinone, it is the hydroquinone, or 3 electron form, that is able to donate electrons to the desired redox partners.
As part of the microsomal electron transport system, CYPOR moves electrons from:
- NADPH → FAD → FMN → Cytochrome P450
Specifically a hydride anion is moved from NADPH to the FAD.[2] The two electrons are then individually passed to FMN, in a process that is believed to be conformationally gated.[2] As previously discussed in the structure section, this belief is based upon the fact that electrons do not appear to be able to be transferred from FAD to FMN unless the two ligands are in close proximity.[2] The protein accomplishes this by undergoing a conformational change, believed to occur because of the flexibility of the hinge domain, which brings the flavin isoalloxazine rings of FMN and FAD into close proximity to one another.[2] In this closed conformation van der Waals forces help hold the ligands together, allowing for efficient electron movement.[2] However, for CYPOR to transfer the electrons, again one at a time, from FMN to cytochrome P450, CYPOR cannot be in a closed conformation because it prevents cytochrome P450 from being able to access FMN.[2] As a result CYPOR must undergo another conformational change so it is in an open conformation allowing the necessary surface residues on the FMN binding domain to form interactions with cytochrome P450 for electron transfer to occur (Fig 2).[2] These surface residues have a negative charge
This reduction of cytochrome P450 allows it to function in biosynthesis and biodegradation pathways of a variety of endogenous and foreign hydrophobic substrates, including drugs and steroids.[2][8] Cytochrome b5, cytochrome c and heme oxygenase can also receive electrons from CYPOR.[2] In these cases CYPOR is functioning in the heme degradation pathway, or with monooxygenase and/or 7-dehydrocholesterol reductase in sterol synthesis.[2]
Medical Significance
Studies have shown that the reductase activity of CYPOR is capable of activating anticancer prodrugs.[2] This makes it a potential target for anticancer research and therapy.[2]
References
- ↑ Horecker BL. Triphosphopyridine nucleotide-cytochrome c reductase in liver. J Biol Chem 1950 Apr 1;183(2):593-605
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 Hamdane D, Xia C, Im SC, Zhang H, Kim JJ, Waskell L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J Biol Chem. 2009 Apr 24;284(17):11374-84. Epub 2009 Jan 26. PMID:19171935 doi:10.1074/jbc.M807868200
- ↑ Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: Isolation, characterization, and kinetic studies. J Biol Chem 1962 Aug 1;237:2652-60
- ↑ 4.0 4.1 Smith GC, Tew DG, Wolf CR. Dissection of NADPH-cytochrome P450 oxidoreductase into distinct functional domains. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8710-4. PMID:8078947
- ↑ Li HC, Liu D, Waxman DJ. Transcriptional induction of hepatic NADPH: cytochrome P450 oxidoreductase by thyroid hormone. Mol Pharmacol. 2001 May;59(5):987-95. PMID:11306680
- ↑ Waxman DJ, Morrissey JJ, Leblanc GA. Hypophysectomy differentially alters P-450 protein levels and enzyme activities in rat liver: pituitary control of hepatic NADPH cytochrome P-450 reductase. Mol Pharmacol. 1989 Apr;35(4):519-25. PMID:2495435
- ↑ Ram PA, Waxman DJ. Thyroid hormone stimulation of NADPH P450 reductase expression in liver and extrahepatic tissues. Regulation by multiple mechanisms. J Biol Chem. 1992 Feb 15;267(5):3294-301. PMID:1737785
- ↑ Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure. 1995 Jan 15;3(1):41-62. PMID:7743131
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |