Sandbox 171
From Proteopedia
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. | |||||||||
| |||||||||
| 2mys, resolution 2.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Overview
Myosin is one of three major classes of molecular motor proteins: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. [1]
Crystallization and X-ray diffraction
Myosin is found in abundance, therefore it can be prepared in gram quantities. [2] For nearly 30 years the myosin head was resistant to crystallization yet by 1993 researchers discovered a mechanism to obtain x-ray quality crystals. [2] The process modified the protein by reductive methylation. [2] X-ray data was used to determine the tertiary structure of the protein. [2]
Structure
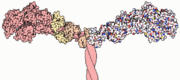
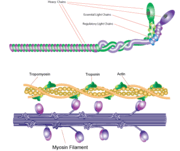
Myosin has a molecular size of approximately 520 kilodaltons, with two 220 kD heavy chains and two pairs of light chains which vary in size.[2] The molecule is asymmetric, having a long tail and two globular heads. [2] Each heavy chains composes the bulk of one of the globular heads. [2] Subfragment-1(S1) also termed the myosin head consists of ATP, actin, and two light chain binding sites.[2] Each globular head has a heavy chain and two light chains for a combined molecular size of about 130 kD. [2]
The myosin head is assymetrical with a length of 165 Angstroms and 65 Angstroms in width, with a total thickness of about 40 Angstroms. [2] About 48% of the amino acid residues in the myosin head are dominated by α helices. [2] One long α helix of about 85 Angstroms stretches from the thick part of the myosin head to the COOH-terminus of the heavy chain. [2] This particular helix forms the light chain binding region on the heavy chain. [2]
Function
Click the link to access DNAtube video "A Moving Myosin Motor Protein" http://www.dnatube.com/video/389/A-Moving-Myosin-Motor-Protein-myosin-actin-interaction
Literature Cited
- ↑ Spudich JA, Finer J, Simmons B, Ruppel K, Patterson B, Uyeda T. Myosin structure and function. Cold Spring Harb Symp Quant Biol. 1995;60:783-91. PMID:8824453
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:8316857