Calcium-free Calmodulin
From Proteopedia
Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Chris Truscott
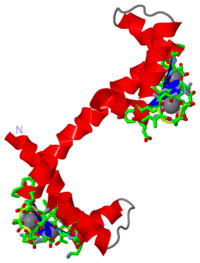
Calcium-free Calmodulin
General InformationCalmodulin is a molecule that has been studied extensively in its functions within the cell, and has an important role in relaying Ca2+ signals within the cytosol. [1] It does this by binding to Ca2+, undergoing a conformational change, and may interact with various proteins within the cell.[1][2][3] It consists of 148 residues and is somewhat shaped like a dumbell, where one end is an N-terminal domain, the other end is the C-terminal domain, and they are connected by a link of about 5 residues. [2] It is also part of the "EF hand" superfamily, named after the pair of helix-loop-helix motifs present in its N and C domains. [2] Once bound to a target protein, it undergoes a further conformational change and may activate certain systems. For example, there is a Ca2+ pump in the plasma membrane pump that is activated by the binding of Ca2+-bound calmodulin, and then uses ATP to drive the Ca2+ out of the cell. [4] Calcium-bound CalmodulinThe structure of calcium-bound calmodulin had previously been discovered using x-ray crystallography. [3] It was then theorized that knowledge of the structure of calcium-free calmodulin would give greater insight into the function of the protein. Attempts were made to crystallize the calcium-free (or apo) form, but to no avail, thus it was decided that the only way to get a good idea of the structure would be to use several NMR experiments. [2]
Calcium-free CalmodulinThe structure of calcium-free calmodulin was discovered by doing several different NMR experiments that included ROE, reverse labelling of Phe residues, and 3-bond J-couplings. [2] It was theorized that by comparing the results of the NMR experiments for the apo calmodulin with the calcium-bound calmodulin, information could be gleaned as to the structural changes that occur when calcium is bound. It was discovered that the series of residues from Met76 to Ser81 which comprised the flexible link between the N-terminal domain and the C-terminal domain were in a helical form in apo calmodulin, but not in calcium-bound calmodulin. [2] These findings suggested that when calcium binds to both domains on calmodulin, a conformational change occurs that involves the unwinding of this helical arrangement. [2] It was further inferred that when calcium binds to calmodulin, the resulting conformational change creates hydrophobic pockets on the surface of the N and C domains which were not present in the apo state. [2][5] There are proteins with similar structures to calmodulin, and troponin was in fact used as a comparison model, as it was highly homologous (51% sequence identity)to calmodulin. [2]
References
|
|||||||||||||||||||||||||||||||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Christopher Truscott, David Canner, Alexander Berchansky, Michal Harel, Andrea Gorrell