User:Satyan Sharma/Sandbox 1
From Proteopedia
|
Introduction
Thiolase is best known as key enzyme in the β-oxidation of fatty acid degradation pathway. However, in the human metabolism at least six thiolases have been characterized. Each of them is important in different pathways, and in different organelles. Some of the thiolases are dimers, some thiolases are tetramers, being dimers of dimers. Thiolases are involved, either in degradative pathways (E.C.2.3.1.16), or in biosynthetic pathways (E.C.2.3.1.9). This division is somewhat arbitrary as all thiolases are aequence related to each other.
Figure 1 shows the thiolase reaction in the degradative direction. In the degradative reaction acetoacetyl-CoA is degraded with CoA as cosubstrate and by which two molecules of acetoacetyl-CoA are formed. Each thiolase catalyzes this reaction in both directions, but the equilibrium is far much favouring the degradative direction (Keq is 105, favoring the degradation). The reaction is catalysed by the enzyme as a two step process. In the first step a fully conserved active site cystiene, Cys89 is acetylated liberating acetyl-CoA. In the second step the acetyl-moiety is transferred to CoA (Figure 1).
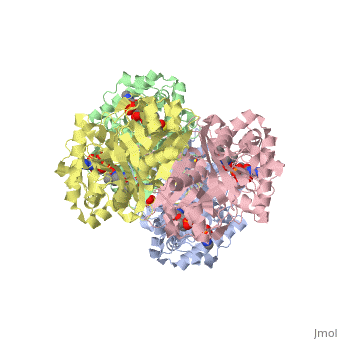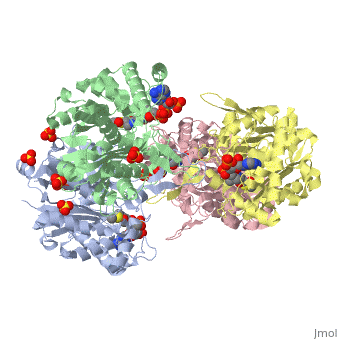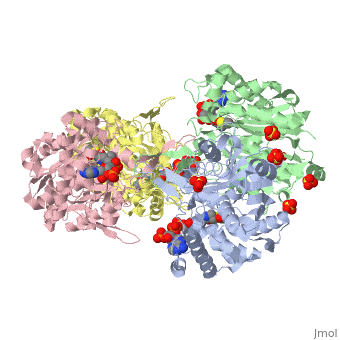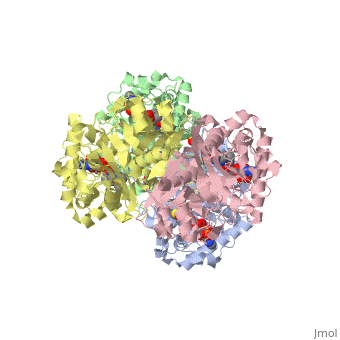
The best studied thiolase is the Zoogloea ramigera thiolase, which is a biosynthetic thiolase and catalyzes the formation of actoacetyl-CoA from two molecules of acetyl-CoA (Figure 2). The enzymological properties of Zoogloea ramigera thiolase have been extensively characterized by C.T. Wals and coworkers(REF-1) and more recently its structural enzymologiacl properties have also been characterized. (REF-2).
The reaction mechanism
The complete catalytic cycle of the Zoogloea ramigera thiolase, is shown in Figure 3. This thiolase is a tetramer, indeed a dimer of dimers. The is deeply buried. It is constructed by four loops, which provide the catalytic nucleophile (Cys89), the base (Cys378), as well as Asn316 and His348.Asn316 and His348 are key residues of the active site geometry: a Asn316-Wat82 diad, together with His348 makes oxyanion hole 1 (green), stabilizing the CoA-thioester enolate intermediate. This enolate is formed on proton abstraction by the catalytic base, Cys378, from acetyl-CoA (Figure 5). His348 has a dual role, as it also activates Cys89 for nucleophilic attack. Further key element of the active site geometry is oxyanion hole 2 (green), which stabilizes the tetrahedral intermediate thioester oxyanion, which is formed when the C2-atom of acetyl-CoA reacts with the carbonyl-atom of the acetyl-Cystiene thioester (Figure 6). The importance of the Asn316-Wat82 diad and His348 for the oxyanion hole 1 stabilization of the transition state in the Claisen condensation reaction has been confirmed by recent studies by Merilainen et. al. (Ref-3).
These key elements of the thiolase reaction mechanism have been capture in the 1DM3 crystal structure (Ref-4), which is a complex of acetyl-CoA complexed with the acetylated enzyme (green). This crystal form is grown at pH 5. At this pH the catalytic base is predicted to be always protonated, independent of the ligand bound in the active site. Therefore it is unable to abstract the proton from the methyl group of acetyl-CoA, and therefore the reaction intermediate, as shown in Figure 7, has been captured.
There are several structures known Zoogloea ramigera thiolase complexed with the intermediates of the reaction cycle (Ref-2). It is interesting to point out that no gross structural changes of loops or side chains have been detected. For example, the only gross structural change with the apo structure (1DLU) is the small movement of the Cys89 side chain towards the His348 side chain, by which it is assumed that Cys89 is deprotonated and therefore activated.