| PPAR</scene>" align="right"/>
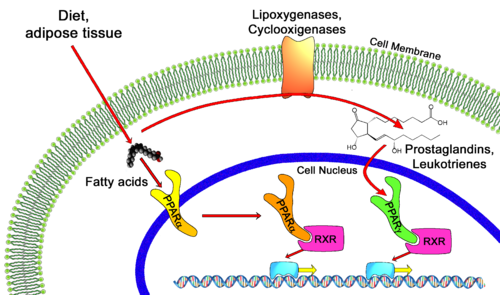
PPARγ binds polyunsaturated fatty acids like linoleic acid, linolenic acid, and eicosapentaenoic acid at affinities that are in line with serum levels. PPARα binds a variety of saturated and unsaturated fatty acids including palmitic acid, oleic acid, linoleic acid, and arachidonic acid.[8] PPARδs ligand selectivity is intermediate between that of the other isotypes and is activated by palmitic acid and a number of eicosanoids.[9]
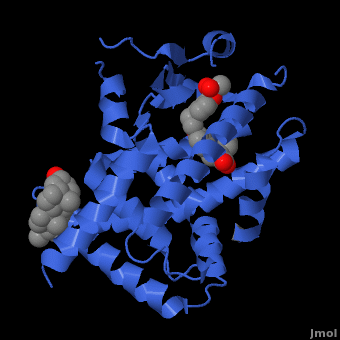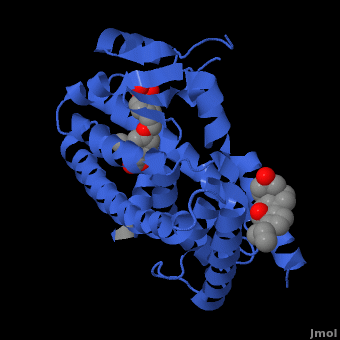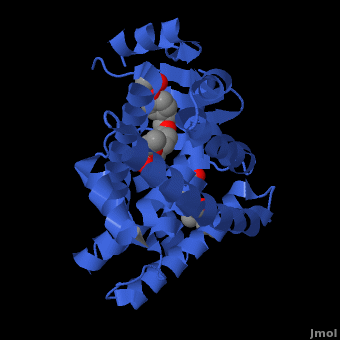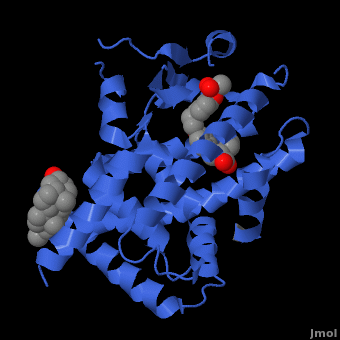
 Human PPARγ Ligand Binding Site with Rosiglitazone Bound. 2prg PPAR Structure
Ligand Binding Domain
The structures of the PPARs are very similar over each isotype. All PPAR isotypes have a ligand binding domain (LBD). The LBD, which is located in the C-terminal half of the receptor, is composed of 13 α-helices and a four-stranded δ-sheet. is Y-shaped and consists of an . The ligand binding pocket of PPARs is quite large in comparison to that of other nuclear receptors (about 1400 cubic angstroms) which allows the PPARs to interact with a broad range of structurally distinct ligands.[10]. Within Arm I, four polar resides are conserved over all PPAR isotypes, in the case of PPARα. These residues are part of a hydrogen bonding network that is formed with the carboxylate group of fatty acids and other ligands upon binding.[11] The , whose function is to generate the receptors’ co-activator binding pocket is located at the C-terminal end of the LBD.[12] The conserved hydrogen bonding network in , promoting co-activator binding.[13] and is thus ideal for binding the hydrophobic tail of fatty acids via Van der Waals interactions.
Despite over 80% of the ligand binding cavity residues being conserved over all PPAR isotypes, it is the remaining 20% that creates the ligand specificity seen between isotypes. A few examples illustrate this point. In PPARδ, the cavity is significantly narrower adjacent to the AF-2 helix and Arm I. This prevents PPARδ from accommodating large headed TZDs and L-tyrosine based agonsists. In the case of PPARα, PPARα does not bind ligands with large carboxylate head groups because of as compared to PPARγ which has a smaller equivalent residue in His323.[14] Or in the case of binding some benzenesulfonamide derivatives, the in the case of PPARγ is lost in PPARα (Ile354) and PPARδ(Ile 363)[15]
AF-2 Domain: Structure and Function
As briefly mentioned before, the AF-2 domain is essential for ligand binding and PPAR function. Helix H12 of AF-2 closes on the ligand-binding site upon ligand binding, reducing conformational flexibility of the LBD and assuming a structure that is ideal for co-activator binding. Using Molecular Dynamic simulations, it has been determined that residues **Glu324, Arg397, Arg443, and Tyr 447** (in PPARγ) are involved in a hydrogen-bond network that stabilizes the AF-2 helix in the active conformation upon ligand binding.[16]
Co-Activator & Co-Repressor Binding
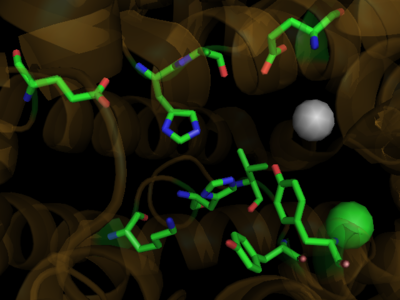
 Human PPARγ Co-Activator Binding Site. PPARγ bound to SRC-1, 3dzyThe transcriptional activity of PPAR is regulated by its interaction with co-activators like SRC-1 or CBP and co-repressors like SMRT. [17]Co-activators like CBP contain a conserved LXXLL motif where X is any amino acid, and use this to bind a hydrophobic pocket on the receptor surface formed by the stabilized AF-2 helix H12.[18] In the case of the PPARγ/rosiglitazone/SRC-1 complex, the hydrophobic face of the LXXLL motif helix of SRC-1 forms **hydrophobic interactions with Leu468 and L318 of the LBD and hydrogen bonds between Glu471 and Lys301 and the co-activator backbone.** These charged residues are conserved across PPAR isotypes and form the “charge clamp,” an essential component for co-activator stabilization in the PPAR LBD.[19]
When PPAR is bound to a co-repressor, the **hydrogen bond between Tyr 464 (in PPARα) and AF-2 is destroyed**, preventing the AF-2 H12 helix from occupying its active state. This in turn eliminates the charge clamp between PPAR and a prospective co-activator.[20]
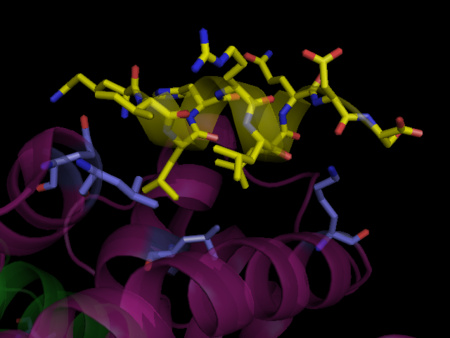
Formation of Heterodimer with RXR
The interface of PPAR and RXR is composed of an intricate network of hydrophobic and polar interactions that shows remarkable complementarity. For the PPARγ-RXRα dimer the **dimmer interface interactions are particularly extensive.** [21]
DNA Binding Domain Structure
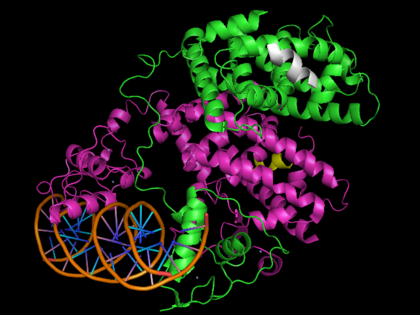
PPARs also contain a **DNA binding domain (DBD)** The DBD consist of **two zinc fingers**, one on PPAR and one on RXR, that bind PPREs of PPAR-responsive genes. The consensus sequence of PPREs is AGGTCA and has been found in a number of PPAR inducible genes like acyl-CoA oxidase and adipocyte fatty acid-binding protein.[22] It is believed that the DNA PPRE actually allosterically contributes to its own binding by directing a head-to-tail interaction between the PPAR DBD and RXR DBD via residues Gln206 and Arg209 on RXRα and Asn160 on PPARγ.[23]
Binding of Synthetic Agonists and Medical Implications
A number of synthetic agonists have been developed to bind to PPAR to fight metabolic diseases like diabetes. These agonists include troglitazone ([www.rezulin.com Rezulin]), pioglitazone ([www.actos.com Actos]), and rosiglitazone ([www.avandia.com Avandia)). These agonists function in a similar fashion, by binding to the active site of PPARγ, activating the receptor. Rosiglitazone occupies roughly 40% of the LBD. It assumes a U-shaped conformation with the TZD head group forming a **number of interactions that stabilize the agonist**. Rosiglitazone forms hydrogen bond interactions with H323 and H449 and its TZD group, the sulfur atom of the TZD occupies a hydrophobic pocket formed by Phe363, Glu286, Phe282 and Leu469, and the central benzene ring of the ligand occupies a pocket formed by Cys285 and Met364.[24]
 Human PPARα agonist, Ciprofibrate (Modalim) Despite their structural similarities, each member of the PPAR family is localized to certain parts of the body. Location of receptor partially determines their function in the body and also the different roles they can play in medicine as drug targets. PPARγ is responsible for lipid metabolism and cellular energy homeostasis. It binds genes that transcribe proteins which act as fatty acid transporters, are critical in insulin signaling and glucose transport, catalyze glycerol synthesis from triglycerides, and catabolize lipids. This makes PPARγ an ideal target to treat Diabetes.[25] Also, recent research has indicated that some PPAR agonists like Rosiglitazone can induce apoptosis of macrophages and would thus serve as excellent anti-inflammatory targets. [26] PPARα has been shown to play a critical role in the regulation of cellular uptake and oxidation of fatty acids. This makes PPARα an excellent target for Atherosclerosis drugs which aim at reducing LDL cholesterol and increasing HDL cholesterol, the two most common traits of atherosclerosis. The fibrates are a class of amphipathic carboxylic acids that are PPARα agonists used to treat hypercholesterolemia and hyperlipidemia along with the HMGR inhibitor statins . Some fibrates are Bezafibrate (Marketed by Roche as Bezalip) and Ciprofibrate (Modalim).[27] PPARδ is broadly expressed across the human body and thus is suspected to play a role in a number of diseases. It has been implicated in disorders ranging from Fertility problems to types of cancer. Perhaps the most important use of PPARδ agonists will be in treating central nervous system (CNS) diseases as PPARδ has been implicated in neuron myelinogenesis and neuronal signaling as well as lipid metabolism in the CNS. [28]
Most drugs target the PPARγ LBD, as ligands that bind to RXRα are likely to inadvertently act on other RXRα complexes, resulting in unexpected side effects. [29] Sales of Avandia, marketed by GlaxoSmithKline peaked at $2.5 billion in 2006 but have dipped dramatically since due to health concerns. In response to the health concerns, sales of Actos, marketed by Takeda, have grown to block buster status.[30]
Additional 3D Structures of PPAR
PPARγ Structures
2zk0, 2zk1, 2zk2, 2zk3, 2zk4, 2zk5, 2zk6 – Crystal Structure of PPARγ bound to various ligands
2prg – Crystal Structure of PPARγ bound to Rosiglitazone and SRC-1
3prg – Crystal Structure of PPARγ
4prg – Crystal Structure of E. Coli PPARγ bound to 2,4-thiazolidinedione derive.
1fm9 – Crystal Structure of PPARγ bound to GI262570, Farglitazar and SRC-1
1fm6 – Crystal Structure of PPARγ bound to Rosiglitazone and SRC-1
1wm0 – Crystal Structure of PPARγ bound to2-BABA and GRIP-1
3ho0, 3hod – Crystal Structure of PPARγ bound to aryloxy-3phenylpropanoic acid
1k74 – Crystal Structure of PPARγ bound to GW409544 and SRC-1
3et0 – Crystal Structure of PPARγ bound to a propionic acid moiety
1knu – Crystal Structure of PPARγ bound to Carbazole analogue
1i7i – Crystal Structure of PPARγ bound to AZ242
2fvj – Crystal Structure of PPARγ bound to Isoquinoline derivative and SRC-1
2g0g – Crystal Structure of PPARγ bound to Pyrazol-5-ylbenzenesulfonamide derivative.
1nyx – Crystal Structure of PPARγ bound to Ragalitazar
1rdt – Crystal Structure of PPARγ bound to GI262570, Fraglitazar and CBP
1zgy – Crystal Structure of PPARγ bound to Rosaglitazone and SHP
2f4b – Crystal Structure of PPARγ bound to Indol-1-yl Acetic Acid Derivative
3lmp – Crystal structure of PPARγ bound to a cercosporamide derivative modulator
PPARα Structures
1k7l – Crystal Structure of PPARα bound to G2409544 and SRC-1
3e94 – Crystal Structure of PPARα bound to tributyltin
1i7g – Crystal Structure of PPARα bound to AZ242
1kkq – Crystal Structure of PPARα bound to GW6471 Antagonist and SMRT
PPARδ Structures
2baw, 2b50, 2awh – Crystal Structure of PPARδ bound to Vaccenic Acid
1gwx – Crystal Structure of PPARδ bound to GW2433
2gwx – Crystal Structure of PPARδ
3gwx – Crystal Structure of PPARδ bound to 5,8,11,14,17-Eicosapentaenoic Acid
1y0s – Crystal Structure of PPARδ bound to GW2331
3dy6 – Crystal Structure of PPARδ bound to anthranilic acid
3et2 – Crystal Structure of PPARδ bound to 3-[5-Methoxy-1-(4-methoxy-benzenesulfonyl)-1H-indol-3-yl]-propionic acid
References
- ↑ Tan NS, Michalik L, Desvergne B, Wahli W. Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem Mol Biol. 2005 Feb;93(2-5):99-105. PMID:15860251 doi:10.1016/j.jsbmb.2004.12.025
- ↑ Qi C, Zhu Y, Reddy JK. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem Biophys. 2000;32 Spring:187-204. PMID:11330046
- ↑ Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005 Feb 15;19(4):453-61. Epub 2005 Jan 28. PMID:15681609 doi:10.1101/gad.1263305
- ↑ Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995 Jun;15(6):3012-22. PMID:7539101
- ↑ Lee SK, Jung SY, Kim YS, Na SY, Lee YC, Lee JW. Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2. Mol Endocrinol. 2001 Feb;15(2):241-54. PMID:11158331
- ↑ Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999 Jun 25;284(5423):2174-7. PMID:10381882
- ↑ Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7473-8. PMID:10377439
- ↑ Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653-7. PMID:1316614
- ↑ Amri EZ, Bonino F, Ailhaud G, Abumrad NA, Grimaldi PA. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem. 1995 Feb 3;270(5):2367-71. PMID:7836471
- ↑ Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998 Sep 10;395(6698):137-43. PMID:9744270 doi:10.1038/25931
- ↑ Fyffe SA, Alphey MS, Buetow L, Smith TK, Ferguson MA, Sorensen MD, Bjorkling F, Hunter WN. Recombinant human PPAR-beta/delta ligand-binding domain is locked in an activated conformation by endogenous fatty acids. J Mol Biol. 2006 Mar 3;356(4):1005-13. Epub 2006 Jan 4. PMID:16405912 doi:10.1016/j.jmb.2005.12.047
- ↑ Yang W, Rachez C, Freedman LP. Discrete roles for peroxisome proliferator-activated receptor gamma and retinoid X receptor in recruiting nuclear receptor coactivators. Mol Cell Biol. 2000 Nov;20(21):8008-17. PMID:11027271
- ↑ Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta. 2007 Aug;1771(8):915-25. Epub 2007 Jan 18. PMID:17317294 doi:10.1016/j.bbalip.2007.01.007
- ↑ Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta. 2007 Aug;1771(8):915-25. Epub 2007 Jan 18. PMID:17317294 doi:10.1016/j.bbalip.2007.01.007
- ↑ Lu IL, Huang CF, Peng YH, Lin YT, Hsieh HP, Chen CT, Lien TW, Lee HJ, Mahindroo N, Prakash E, Yueh A, Chen HY, Goparaju CM, Chen X, Liao CC, Chao YS, Hsu JT, Wu SY. Structure-based drug design of a novel family of PPARgamma partial agonists: virtual screening, X-ray crystallography, and in vitro/in vivo biological activities. J Med Chem. 2006 May 4;49(9):2703-12. PMID:16640330 doi:10.1021/jm051129s
- ↑ Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995 Dec 14;378(6558):681-9. PMID:7501014 doi:http://dx.doi.org/10.1038/378681a0
- ↑ Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta. 2007 Aug;1771(8):915-25. Epub 2007 Jan 18. PMID:17317294 doi:10.1016/j.bbalip.2007.01.007
- ↑ Gampe RT Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000 Mar;5(3):545-55. PMID:10882139
- ↑ Nagy L, Schwabe JW. Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci. 2004 Jun;29(6):317-24. PMID:15276186 doi:10.1016/j.tibs.2004.04.006
- ↑ Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, Oplinger JA, Kliewer SA, Gampe RT Jr, McKee DD, Moore JT, Willson TM. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13919-24. Epub 2001 Nov 6. PMID:11698662 doi:10.1073/pnas.241410198
- ↑ Gampe RT Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000 Mar;5(3):545-55. PMID:10882139
- ↑ Wahli W, Braissant O, Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more.... Chem Biol. 1995 May;2(5):261-6. PMID:9383428
- ↑ Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008 Nov 20;456(7220):350-6. PMID:19043829 doi:10.1038/nature07413
- ↑ Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998 Sep 10;395(6698):137-43. PMID:9744270 doi:10.1038/25931
- ↑ Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-35. PMID:11818483 doi:10.1146/annurev.med.53.082901.104018
- ↑ Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998 Oct 2;273(40):25573-80. PMID:9748221
- ↑ Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-35. PMID:11818483 doi:10.1146/annurev.med.53.082901.104018
- ↑ Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-35. PMID:11818483 doi:10.1146/annurev.med.53.082901.104018
- ↑ Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008 Nov 20;456(7220):350-6. PMID:19043829 doi:10.1038/nature07413
- ↑ http://uk.reuters.com/article/idUKT7482820080131
|