Ketosteroid Isomerase
From Proteopedia
Contents |
Ketosteroid Isomerase
Introduction
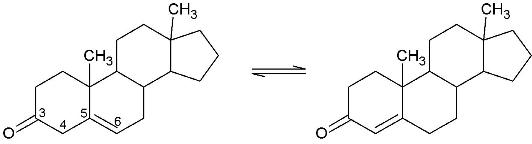
Template:STRUCTURE 1isk (KSI, EC#5.3.3.1) is an enzyme that catalyzes the isomerization of 3-oxo-Δ5 ketosteroids to their hormonally active Δ4-conjugated isomers, as illustrated below.[1], [2]
This reaction is essential in the biosynthesis of steroids in mammals where KSI is a membrane-bound complex.[3] In bacteria, however, KSI exists as a soluble protein is involves in catabolism of steroids.[3] It was first isolated in and has been extensively studied in Commamonas tetosteroni (TI), a bacteria that is capable of growth with testosterone as its sole carbon source.[4] Structural and kinetic studies of this and its homolog from Pseudomonas putida with which it shares 34% sequence and near identical structural homology.[1],[3] It is one of the most efficient known enzymes with an essentially diffusion limited rate of catalysis.[2],[5] It is capable of increasing the catalytic rate by eleven orders of magnitude.[6] The high degree of efficiency is believed to be due to a preference for the transition state to move towards products rather than reactants although the exact mechanism of this preference is unclear.[1] Its high catalytic efficiency and unique active site geometry have made it fertile ground for examining the validity of the low barrier hydrogen bond hypothesis[7] and electrostatic preorganization.[8].
Structure
Ketosteroid isomerase exits as a 28 kDa homodimeric protein, in which the two dimers related to each other via hydrophobic and electrostatic interactions.[5] Each monomer consists of a curved and three . These secondary structures define a conical closed barrel geometry, with one open and one closed end, and create a deep pocket in which the active site resides.[3],[9] This unique geometry is shared by several other proteins (scytalone dehydratase, nuclear transport factor 2, and naphthalene 1,2-dioxygenase), however, these molecules do not share functional or sequence homology. It is speculated that this unique protein structure may enable better binding of hydrophobic substrates such as steroids.[3]
Alpha-Helices
Each monomer of KSI contains three α-helices. is contains residues Thr3 to (N-cap) to Ala20. contains residues Asp22(N-cap) to Phe30. contains residues Thr48 to Leu61. [5]
Alpha-Helix Capping Motifs
Alpha-Helix Packing
Beta-Sheet
Hydrophobic Active Site
KSI's active site is located within a hydrophobic cavity formed helices B and C crossing over the "front face" of the β-sheet that is approximately 8.5 by 9.5 Å at its opening and is 16 Å deep.[5] The cavity is lined with from the β-sheet(Val36, Pro39, Leu63, Val65, Leu67, Val71, Phe80, Phe82, Val84, Val95, Pro97, Phe101, Ala114, and Phe116). contributed from the α-helices include: Val11, Tyr14, Val15, Leu18, Phe54, and Tyr55.[5]
Although the of KSI is notably hydrophobic, it contains several hydrophilic residues believed to be important to the enzymatic function of the protein. The hydrophobic active site of KSI contains an aspartate residue at position 99 and a tyrosine residue at position 14 (according to the numbering for the Commamonas tetosteroni protein, which will be used throughout) that are capable of forming hydrogen bonds with the 3-position carbonyl of the steroid and form an active site oxyanion hole.[1],[10] Additionally, the active site contains an aspartate residue at position 38 that is participates in the catalytic activity of KSI.[1]Cis-Peptide Bond
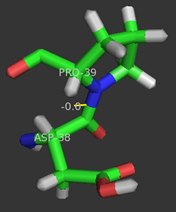
Pro39 of KSI participates in a cis-peptide linkage with Asp38 in forming a between stands 1 and 2 of the 6 stranded beta-sheet.[5] The cis-peptide linkage serves to correctly position the key catalytic residue Asp38 within the active site. Mutating residue to Gly or Ala results in the improper positioning of Asp38 within the active site leading to corresponding 2-fold decreases in enzyme's catalytic efficiency.[11]
Structural Classifications
CATH
KSI is classified using the CATH classification system as follows:
- Class - Alpha Beta
- Architecture - Roll
- Topology - Nuclear Transport Factor 2
SCOP
KSI is classified using the SCOP classification system as follows:
- Class - Alpha and Beta (α+β)
- Fold - Cystatin-like
- Superfamily - NTF2-like
- Family - Ketosteroid Isomserase-like
- Domian - Δ5-3-ketosteroid isomerase
Enzyme Mechanism
General Mechanism
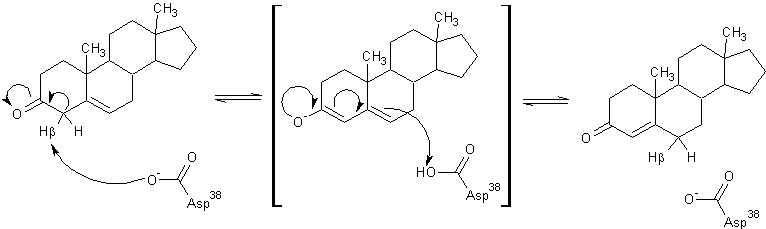
The general mechanism of the proposed reaction of ketosteroid isomerase involves the breaking of a C-H bond adjacent to a carbonyl. This is typically regarded as a difficult reaction due to instability of the intermediate; however it is observed in a number of enzyme-mediated biological reactions.[1] In line with other biological reactions, the mechanism of KSI involves the abstraction the β-hyrdogen from the 4-position carbon resulting in the formation of an enol intermediate, which is followed by reketonization.[1],[3] Structural and kinetic studies suggest that Asp38 (numbering is that of the TI varient of KSI) serves as a general base in this reaction as shown below and abstracts the β-proton from C4 with the syn orbitals of its carboxylate group.[5] Note the formation of the unstable enolate intermediate.
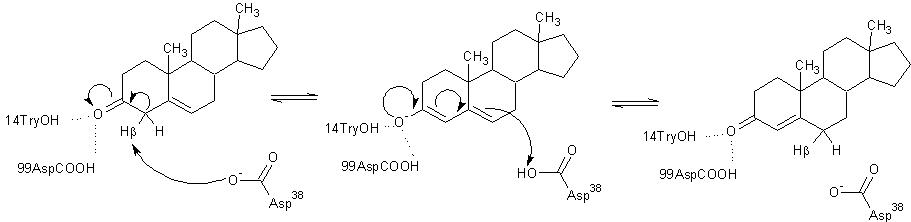
Tyr14 and Asp99 are believed to participate in hydrogen bonding to the O-3 carbonyl of the substrate steroid and stabilize reaction intermediates. Tyr14 is also believed to participate in a low barrier hydrogen bond with the 3-position oxygen of the steroid, thereby facilitating the abstraction of the β-hydrogen at the 4-position. There are two proposed models of this hydrogen bonding. In , Tyr14 and Asp99 are both bound to the 3-position oxygen, whereas, in , they form a hydrogen bonding network. These are as a single monomer in complex with the intermediate analog equilenin.[12]
Model 1 has become the generally accepted scheme through both crystallographic and mutanagenic/kinetic approaches. Mutation of Asp99 to alanine and Tyr14 to phenylalanine resulted in deleterious effects on kinetic parameters, which were additive in nature suggesting that Tyr14 and Asp99 participate equally in hydrogen binding with the oxygen atom. Additionally, the crystal structure of TI analog from Pseudomonas putida supports this conclusion given the .[1] The general mechanism in the active site of KSI is outlined below.
Low Barrier Hydrogen Bond
Ketosteroid isomerase has become a test enzyme in the debate over the existence of low barrier hydrogen bonds (LBHBs) in accordance with the hydrogen bonding discussed above. Nuclear magnetic resonance (NMR) studies of KSI in complex with transition state analogs have revealed the presence of a highly shielded proton characteristic of the formation of a LBHB between Tyr14 and the O-3 atom of the analogs and suggestive of the formation of a bridging hydrogen with short bonds.[3],[13],[7] Additionally, NMR fractionation studies with deuterium substitution are strongly suggestive of the high strength of this bond as deuterium is retained preferentially in this position.[13] The energy of this bond along with that of the normal hydrogen bond from Asp99 most likely contribute to the energy needed to support proton abstraction from the C-4 position.[3]
LBHB Controversy and Catalytic Efficiency from Preorganized State
In their 2002 computational study Feierberg and Aquist propose that KSI is not the site of LBHB mediated catalysis[14], a viewpoint that is shared among a subset of enzymologists.[15] Hershlag and coworkers are proponents of the hydrogen bonding mechanism described in ;[15],[16] however, he suggests that hydrogen bonding is important within the active site of KSI albeit in the form of multiple weaker forces.[8] Hydrogen bonds and other electrostatic contributions are contended to allow for a degree of preorientation of the active site for transition state stabilization that helps to counter act the effects of desolvation and reorientation of dipoles within the enzyme active site to support the oxyanion hole, which helps to avoid "sluggish" behavior associated with such rearrangement.[1] This type of hydrogen bonding network also helps to disperse negative charge enabling stabilization of the transition state because in the hydrophobic environment of the active site large charges are abhorred.[8]
Implications of Hydrophobic Active Site
In combination with the above mentioned hydrogen bond interactions between the substrate and the active site, the highly hydrophobic active site also serves to stabilize the catalytic efficiency of KSI because it is in a preorganized state compared to the of the reaction carried out in solvent where acetate anion is used to mimic the functionality of Asp38 and has a similar pKaof 4.6 as suggested above. The consequence of this hydrophic active site is that Asp38 has no neighbors capable of hydrogen bonding interactions and as such one may suspect its pKa to raised in this environment. Maintenance of this normal pKa is important because protonation of Asp38 would no longer permit it to carry out hydrogen abstraction. To prevent divergence of Asp38 from its normal pKa during initial substrate binding, it is believe hydrogen bonded to two or three water molecules, as suggested by computer models, which are excluded from the active site later in the enzymatic reaction.[1]
Related Proteins
Functionally Related Proteins
Ketosteroid isomerase is one of a large number of enzymes that catalyze the cleavage of a C-H bond. Among these are mandelate racemace, triosephosphate isomerase, citrate synthase, and 4-oxalocrotonate tautomerase.[1]
Structural Homologs
As noted above, scytalone dehydratase, nuclear transport factor 2 (NTF2), and naphthalene 1,2-dioxygenase share similar structural motifs to ketosteroid isomerase, which facilitate binding of hydrophobic substrates.[3],[6] It has also been postulated that bile acid 7α-dehydratase is also a member of this family of protein and that it shares functional similarity with KSI.[6] In a recent paper, Cherney et al[17]. identified Myobacterium tuberculosis Rv0760c as a structural homolog of KSI. Although these proteins share similar structural motifs there carry out a diverse array of functions.[17]
Available Structures
- 1isk-First solution phase structure NMR structure of KSI from Comamonas testosteroni
- 1opy
- 1buq
- 1qjg-KSI from Pseudomoas testosteroni in complex with equilenin
- 1c7h
- 1e3r
- 1e3v
- 1e97
- 1ea2
- 1k41
- 1gs3
- 1ogx
- 1oh0-KSI from Pseudomonas putida in complex with equilenin
- 1cqs
- 1vzz
- 1w01
- 1w02
- 1oho-KSI Y16F/D40N mutant from Pseudomonas putida in complex with equilenin
- 1w6y
- 1w0o
- 2pzv
- 2inx
- 3cpo
- 3ex9
- 3fzw
- 3ipt
- 3m8c
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Pollack RM. Enzymatic mechanisms for catalysis of enolization: ketosteroid isomerase. Bioorg Chem. 2004 Oct;32(5):341-53. PMID:15381400 doi:10.1016/j.bioorg.2004.06.005
- ↑ 2.0 2.1 TALALAY P, WANG VS. Enzymic isomerization of delta5-3-ketosteroids. Biochim Biophys Acta. 1955 Oct;18(2):300-1. PMID:13276386
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Ha NC, Choi G, Choi KY, Oh BH. Structure and enzymology of Delta5-3-ketosteroid isomerase. Curr Opin Struct Biol. 2001 Dec;11(6):674-8. PMID:11751047
- ↑ Ha NC, Choi G, Choi KY, Oh BH. Structure and enzymology of Delta5-3-ketosteroid isomerase. Curr Opin Struct Biol. 2001 Dec;11(6):674-8. PMID:11751047
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Wu ZR, Ebrahimian S, Zawrotny ME, Thornburg LD, Perez-Alvarado GC, Brothers P, Pollack RM, Summers MF. Solution structure of 3-oxo-delta5-steroid isomerase. Science. 1997 Apr 18;276(5311):415-8. PMID:9103200
- ↑ 6.0 6.1 6.2 Murzin AG. How far divergent evolution goes in proteins. Curr Opin Struct Biol. 1998 Jun;8(3):380-7. PMID:9666335
- ↑ 7.0 7.1 Cleland WW, Frey PA, Gerlt JA. The low barrier hydrogen bond in enzymatic catalysis. J Biol Chem. 1998 Oct 2;273(40):25529-32. PMID:9748211
- ↑ 8.0 8.1 8.2 Kraut DA, Sigala PA, Pybus B, Liu CW, Ringe D, Petsko GA, Herschlag D. Testing electrostatic complementarity in enzyme catalysis: hydrogen bonding in the ketosteroid isomerase oxyanion hole. PLoS Biol. 2006 Apr;4(4):e99. Epub 2006 Mar 28. PMID:16602823 doi:10.1371/journal.pbio.0040099
- ↑ Cho HS, Choi G, Choi KY, Oh BH. Crystal structure and enzyme mechanism of Delta 5-3-ketosteroid isomerase from Pseudomonas testosteroni. Biochemistry. 1998 Jun 9;37(23):8325-30. PMID:9622484 doi:10.1021/bi9801614
- ↑ Sigala PA, Kraut DA, Caaveiro JM, Pybus B, Ruben EA, Ringe D, Petsko GA, Herschlag D. Testing geometrical discrimination within an enzyme active site: constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole. J Am Chem Soc. 2008 Oct 15;130(41):13696-708. Epub 2008 Sep 23. PMID:18808119 doi:10.1021/ja803928m
- ↑ Nam GH, Cha SS, Yun YS, Oh YH, Hong BH, Lee HS, Choi KY. The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ketosteroid isomerase from Comamonas testosteroni. Biochem J. 2003 Oct 15;375(Pt 2):297-305. PMID:12852789 doi:10.1042/BJ20030263
- ↑ Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH. Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization. J Biol Chem. 1999 Nov 12;274(46):32863-8. PMID:10551849
- ↑ 13.0 13.1 Zhao Q, Abeygunawardana C, Talalay P, Mildvan AS. NMR evidence for the participation of a low-barrier hydrogen bond in the mechanism of delta 5-3-ketosteroid isomerase. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8220-4. PMID:8710850
- ↑ Zhao Q, Abeygunawardana C, Talalay P, Mildvan AS. NMR evidence for the participation of a low-barrier hydrogen bond in the mechanism of delta 5-3-ketosteroid isomerase. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8220-4. PMID:8710850
- ↑ 15.0 15.1 Kraut DA, Sigala PA, Fenn TD, Herschlag D. Dissecting the paradoxical effects of hydrogen bond mutations in the ketosteroid isomerase oxyanion hole. Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):1960-5. Epub 2010 Jan 11. PMID:20080683
- ↑ Sigala PA, Caaveiro JM, Ringe D, Petsko GA, Herschlag D. Hydrogen bond coupling in the ketosteroid isomerase active site. Biochemistry. 2009 Jul 28;48(29):6932-9. PMID:19469568 doi:10.1021/bi900713j
- ↑ 17.0 17.1 Cherney MM, Garen CR, James MN. Crystal structure of Mycobacterium tuberculosis Rv0760c at 1.50 A resolution, a structural homolog of Delta(5)-3-ketosteroid isomerase. Biochim Biophys Acta. 2008 Nov;1784(11):1625-32. Epub 2008 Jun 6. PMID:18589008 doi:10.1016/j.bbapap.2008.05.012
Proteopedia Page Contributors and Editors (what is this?)
Laura M. Haynes, Michal Harel, Joel L. Sussman, Alexander Berchansky