From Proteopedia
proteopedia linkproteopedia link
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada.
|
To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
|
Thymidylate Synthase
Thymidylate Synthase is a protein found in all organisms that make DNA. Thymidylate Synthase (TS) is the essential enzyme that catalyzes the formation of dTMP from dUMP, using 5,10-methylenetetrahydrofolate (mTHF) as a cosubstrate [1].
Overview
Thymidylate Synthase catalyzes the reductive methylation of deoxyuridylic acid during the de novo synthesis of thymidylic acid [2]. This reaction occurs primarily during the S phase of the cell cycle. Thymidylate synthase is an essential enzyme in proliferating cells that are not supplied with an alternate source of thymidine nucleotides [2]. Research has shown that thymidylate synthase enzyme levels are much higher in rapidly proliferating cells than in non dividing cells [2].
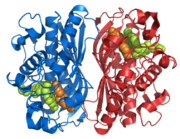
Figure 2. Thymidylate Synthase
Mammalian Thymidylate Synthase
Human and other mammalian thymidylate synthase enzyme have a N-terminal extension of aprox 27 amino acids [3]. This extension is not present in bacterial thymidylate synthase [3]. The extension is believed to play a primary role in protein turnover however not in catalytic activity [3].
Mechanism of Thymidylate Synthase Initiation
Thymidylate synthase converts dUMP to dTMP and is labeled as the rate-limiting enzyme in the synthesis of pyrimidine nucleotides, which are required for DNA synthesis [4]. The conversion of dUMP to dTMP is done with a cosubstrate mTHF [4]. The reaction is initiated by the active site cysteine, Cys195, is attacked by C6 of dUMP and this leads to the formation of C5 of dUMP [4]. The formation generates a second covalent bond between dUMP and mTHF and eventually a ternary catalytic complex [4]. The role of (Figure. 1) in the conversion of dUMP to dTMP can be important to proper structure and interactions that are required to initiate the formation of C5 dUMP.
Significance
The importance of thymidylate synthase as shown in Figure 2, is the essential role it plays in the reproduction of DNA. From a medical prospective, thymidylate synthase has been an important target in the chemotherapy of colon cancer and some other malignancies [1].
References
- ↑ 1.0 1.1 Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Marjorette, M., Peña, O., et al. (2011). Replacement of Val3 in Human Thymidylate Synthase Affects its Kinetic Properties and Intracellular Stability. NIH Public Access, 49(11), 2475-2482. doi: 10.1021/bi901457e.Replacement.
- ↑ 2.0 2.1 2.2 Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.
- ↑ 3.0 3.1 3.2 Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.
- ↑ 4.0 4.1 4.2 4.3 Yamada, H., Ichikawa, W., Uetake, H., Shirota, Y., Nihei, Z., Sugihara, K., et al. (2001). Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clinical colorectal cancer, 1(3), 169-73; discussion 174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450430.


