Sandbox Reserved 332
From Proteopedia
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
Spruce Budworm Antifreeze Protein
|
Introduction
Spruce Budworm antifreeze protein is among a small but growing number of ice structuring proteins present in many cold-dwelling organisms. As suggested by the name, this AFP is found in the Spruce Budworm insect with the highest concentration in the blood plasma. Tissue damage normally caused by mass organelle fracturing during the freezing process at sub-zero temperatures is avoided by the specific action of this unique antifreeze protein (AFPs), allowing survival at temperatures below the standard freezing range. AFPs are measured in their effectiveness to prevent ice crystal growth by the temperature difference between normal melting or freezing point, and the lowest temperature ice crystals remain absent, a phenomenon known as thermal hysteresis, and Spruce Budworm is found at -20 C.
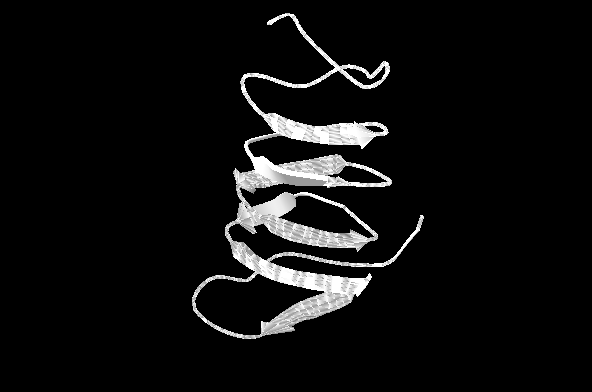
Structure and Function
Spruce Budworm antifreeze protein is a member of the Beta-helix structural family. Monomeric in structure, it consists of a single polypeptide chain in a left handed coil that roughly approximates a triangular prism shape; 28 A in height with 16-17 A base sides. There are four disulphide bridges present and 15 amino acid residues per coil with 10 clearly identifiable beta sheets present in terms of secondary structure with a hydrophobic core. An ice binding face occurs on the side of the protein where four threonine-Xaa-threonine (TXT) motifs are stacked on top of eachother, X being an amino acid of no particular type. A highly geometric, regular pattern is formed by the threonine residues indicative of a likely crystal interaction site, suggesting strongly that the ice binding function relies on this precise structural character of the . It is thought that upon binding of early microscopic ice crystal nucleation sites, sbw AFP promotes the growing crystal to follow an energetically unfavourable crystallization pattern that follows a curve along the solid-liquid phase surface. Sbw AFP therefore does not prevent ice forming at 0 C, but instead limits crystal growth as to prevent catastrophic damage of the cell. N terminus residues form a tail that shows significant conformation heterogeneity between the temperatures 5 C and 30 C with X-ray diffraction data showing incongruities between two generated models.