Sandbox89220
From Proteopedia
Contents |
Phosphomannose mutase 2
Background
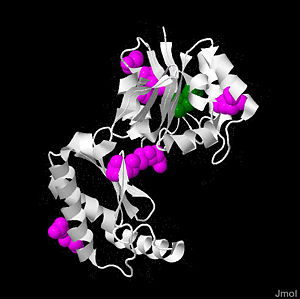
Structure
|
PMM2 is a 246 amino acid long protein, with 2 identical subunits working as independent domains, hence it’s also known as a homodimeric protein. In addition, it’s classified as the member of HAD (haloacid dehydrolase) superfamily due to the fact that it had 4 different types of motifs, each of which is highly conserved across the vertebrates’ lineages during evolutions. Similar to other members in HAD superfamily, the two domains are separately recognized as core and cap domain. The core domain (residues 1 – 90, and 198-262)of PMM2 displays the characteristic 4 motifs of HAD superfamily that contribute to the catalytic ability of the active sites in the protein. Motif 1 has Asp as nucleophile which serves as the mediator for the phosphoryl group transfer, the second Asp, on the other hand, acts as in the general acid-base reaction. While motif 1 provides the nucleophilic and general acid-base reactions, motif 2 in the protein which usually contains Thr or Ser helps in positioning or binding on the substrates’ phosphoryl group. In addition, motif 3 has the typical Lys or Arg residues while motif 4 has the acidic residues (Asp and Glu) that bind magnesium cofactor. The cap domain of PMM2 is smaller, functioning as the regulator for the access of the substrate into the active site of the core domain and at the same time contains the primary and secondary substrate specificity loops that recognize only specific substrate.
How does PMM2 work?
PMM2 converts mannose-6-phosphate into mannose-1-phosphate that would then serve as the building bloc of the dolichol-linked oligosaccharides for glycosylation in endoplasmic reticulum. It's not clear how PMM2 the cap and core domains would coordinate with each other. However,through the extrapolation of its paralogous protein, PMM1 which has a complex mechanism that uses charges of the conserved residues in the cap domains to sweep the substrate into core domain, we could postulate that PMM2 would utilize similar machinery. Also, with the close resemblance to the PGM proteins, PMM2 might have similar machinery that manipulate the conformational changes that “closes” cap domain after binding substrates to allow the specificity loops getting into contact with the active site of the core domain, thus participating in catalysis.
