<Morph showing conformational changes between unbound (1qx3) and inhibitor bound (3kjf).>
and inhibitor of caspase-activated deoxyribonuclease (ICAD) by caspase-3 (Enari, Sakahira et al. 1998; Sakahira, Enari et al. 1998).
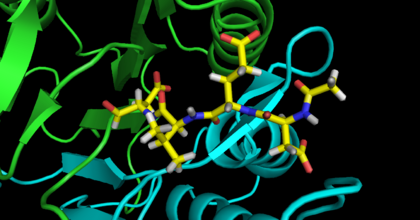
Inhibitor DEVD-CHO bound to Caspase-3,
1PAU
In all three adducts the “warhead” ketone oxygen hydrogen bonds with the backbone amide NH of both the catalytic cysteine and conserved glycine (caspase-3 Gly122). Similarly, the P1 carboxylate moiety forms hydrogen bonds with the side chains of three conserved residues (caspase-3 Arg64; caspase-3 Arg207; caspase-3 Gln161). The adjacent amide nitrogen hydrogen bonds with a main chain carbonyl (caspase-3 Ser205) as well as forming a close contact with the catalytic cysteine sulfur atom (3.0 Å). One of the carbonyls of the urazolopyridazine forms a hydrogen bond with a backbone main chain amide (caspase-3 Arg207). As can be surmised from this enumeration of interactions, the structures show that the core elements of the
inhibitors bind to caspase-3 and caspase-8 in similar ways. These interactions closely mimic those of caspases with peptidic sub- strates (Fig. 8).
Function
(Structural insights into its function)
In its inactive form, caspases consist of a prodomain, large subunit, and small subunit, interjected with aspartic acid residues. These are cleavage sites of which active caspases can cleave leading to an active form in which the subunits come together to form a complex.
Disease
Although the role of caspases in chronic neurodegenerative disease is controversial, their role in acute neurodegenerative disease, such as nerve crush injury and stroke, may be more evident. Inhibition of caspase activity increases survival of neurons.
Studies have also shown that caspases are downregulated to allow for unchecked survival of neoplastic cancers and autoimmune diseases. In particular, caspase-8 has been found to be silenced in neuroblastoma and humans with mutations in caspase-8 and -10 develop ALPS (autoimmune lymphoproliferative syndrome). These raise the possibility that caspase-3 may play a role as well.
Knocking out caspase-3 in 129x1/SvJ mice leads to hydrocephalus and subsequently perinatal lethality. Caspase-3 knockout in C57BL/6J however appear to have no brain abnormalities.
(Fuentes-Prior and Salvesen, 2004)
Evolutionarily Related Proteins
Proteins + (Links to evolutionarily related proteins)
example Caspase 1 through 18
To date, eighteen caspases have been identified. Caspases can largely be grouped into three subfamilies in humans: inflammatory (caspase-1, -4, and -5), effector (caspase-3, -6, and -7), and initiator caspases (-2, -8, -9, and -10). Caspase-11 and -12 substitutes for caspase-4 and -5, respectively, in mice. (Fuentes-Prior and Salvesen, 2004)
Solved Structures
(Links to available structures)
3PCX Caspase-3 E246A, K242A Double Mutant http://www.pdb.org/pdb/explore/explore.do?structureId=3PCX
3PD1 Caspase-3 K242A http://www.pdb.org/pdb/explore/explore.do?structureId=3PD1
3PD0 Caspase-3 E246A http://www.pdb.org/pdb/explore/explore.do?structureId=3PD03KJF
3KJF Caspase-3 bound to a covalent inhibitor http://www.pdb.org/pdb/explore/explore.do?structureId=3KJF
3ITN
3H0E
3GJQ, 3GJR, 3GJS, 3GJT
3EDQ
3DEH, 3DEI, 3DEJ, 3DEK
2CNK, 2CNL, 2CNN, 2CNO, 2CDR
2J30, 2J31, 2J32, 2J33
2C1E, 2C2K, 2C2M, 2C2O
2H51
2H5J
2H65
2DKO, 2CJX, 2CJY
1RE1
1RHJ, 1RHK, 1RHM, 1RHQ, 1RHR, 1RHU
1NME, 1NMQ, 1NMS
1CP3
1PAU
1I3O
1QX3
References & Notes
Alam, A., L. Y. Cohen, et al. (1999). "Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells." J Exp Med 190(12): 1879-90.
Aouad, S. M., L. Y. Cohen, et al. (2004). "Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death." J Immunol 172(4): 2316-23.
Budd, R. C. (2001). "Activation-induced cell death." Curr Opin Immunol 13(3): 356-62.
Degterev, A., M. Boyce, et al. (2003). "A decade of caspases." Oncogene 22(53): 8543-67.
Dohrman, A., J. Q. Russell, et al. (2005). "Cellular FLIP long form augments caspase activity and death of T cells through heterodimerization with and activation of caspase-8." J Immunol 175(1): 311-8.
Enari, M., H. Sakahira, et al. (1998). "A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD." Nature 391(6662): 43-50.
Kennedy, N. J., T. Kataoka, et al. (1999). "Caspase activation is required for T cell proliferation." J Exp Med 190(12): 1891-6.
Koenig, A., J. Q. Russell, et al. (2008). "Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death." Cell Death Differ 15(11): 1701-11.
Li, H., H. Zhu, et al. (1998). "Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis." Cell 94(4): 491-501.
Misra, R. S., D. M. Jelley-Gibbs, et al. (2005). "Effector CD4+ T cells generate intermediate caspase activity and cleavage of caspase-8 substrates." J Immunol 174(7): 3999-4009.
Misra, R. S., J. Q. Russell, et al. (2007). "Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation." J Biol Chem 282(27): 19365-74.
Sakahira, H., M. Enari, et al. (1998). "Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis." Nature 391(6662): 96-9.
Vincent, M. S., K. Roessner, et al. (1996). "Apoptosis of Fashigh CD4+ synovial T cells by borrelia-reactive Fas-ligand(high) gamma delta T cells in Lyme arthritis." J Exp Med 184(6): 2109-17.
Wesselborg, S., O. Janssen, et al. (1993). "Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells." J Immunol 150(10): 4338-45.


