This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Journal:JBIC:11
From Proteopedia

| |||||||||||
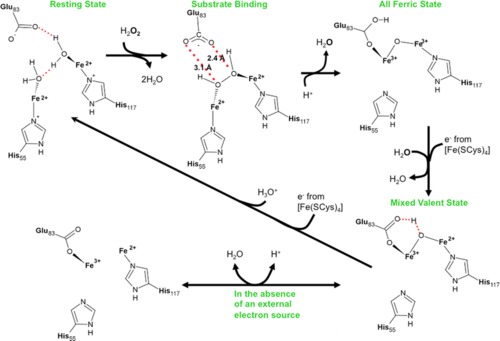
- ↑ Dillard BD, Demick JM, Adams MW, Lanzilotta WN. A cryo-crystallographic time course for peroxide reduction by rubrerythrin from Pyrococcus furiosus. J Biol Inorg Chem. 2011 Jun 7. PMID:21647777 doi:10.1007/s00775-011-0795-6
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.