This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Delta-endotoxin
From Proteopedia
Delta-endotoxin named also Cyt or Cry, is a pore-forming toxin produced by Bacillus thuringiensis. It is used as an insecticide. Upon ingestion by insect, δ-endotoxin is cleaved, binds to the gut epithelium and forms cation channels. This causes cell lysis and death.
|
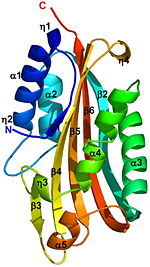
δ-endotoxin from Bacillus thuringiensis
The crystal structure of the proteolytically activated monomeric form of Cyt2Ba was determined at 1.8Å resolution. It consists of a single domain of architecture with a (yellow) surrounded by 2 layers (red) forming a cytolysin fold. The β-sheet is comprised of 6 anti-parallel β-strands (β1-β6). On one side of this sheet there is an α-helix layer consisting of α1, α2; and on the other side a second α-helix layer, composed of α3-α5. The β-strands β2-β5 of the central β-sheet have a modified Greek-key topology. The Greek key motif consists of four adjacent antiparallel strands and their linking loops. It consists of three antiparallel strands connected by hairpins, while the fourth is adjacent to the first and linked to the third by a longer loop [1]. Cyt2Ba (gray) has only 16% sequence identity with VVA2 (colored red, 1pp0), however they both have a cytolysin fold and their overall structure is very similar (see their ). A remarkable similarity is observed between the structures of the endogenously cleaved Cyt2Ba (gray) and the (red) within the inactive protoxin of Cyt2Aa (1cby, monomers A and B of Cyt2Aa shown red and blue, respectively, the N- and C-termini are shown in spacefilling representation). Although, 1cby is a 1 chain structure, the biological relevant molecule for 1cby can be assembled from the contents of the deposited coordinates by the application of crystallographic symmetry operations to give a dimer. It can be downloaded. Each monomer of Cyt2Aa (1cby), consists of an additional β-strand at its N-terminus and an additional α-helix at its C-terminus compared to the cleaved Cyt2Ba. The of Cyt2Aa is held together by the intertwined N-terminal strands from both monomers. The cleavage of Cyt2Aa the N- and C-terminal segments, prevents dimer formation and releases an . Similarly, in Cyt2Ba the proteolysis causes the removal of 34 amino acids at its N-terminus and 28 or 30 residues at its C-terminus forming the crystallized toxic monomer. The crystal structure of monomeric Cyt2Ba is the first structure of a toxic form of the Cyt family. Its structure is homologous to the corresponding region of Cyt2Aa and to that of VVA2. This structural comparison shows that the toxicity of Cyt2Ba, Cyt2Aa and VVA2 is an inherent property of the monomer and not the result of secondary structure rearrangement upon cleavage. Solving the 3D structure of these proteins extends the knowledge of the cytolytic machinery of pore-forming toxins and helps in designing novel membrane-active cytotoxins.
3D Structures of δ-endotoxin
3eb7 – BtCry8Ea1 – Bacillus thuringiensis
2rci – BtCytBa
2c9k – BtCry4A residues 68-679
1w99 - BtCry4Ba residues 84-641
1dlc - BtCryIiia residues 61-644
1ji6 - BtCryIiib residues 64-652
1cby - BtCytB residues 1-259