Ferguson ZNF Sandbox
From Proteopedia
| |||||||
| 1znf, 37 NMR models () | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Non-Standard Residues: | , | ||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
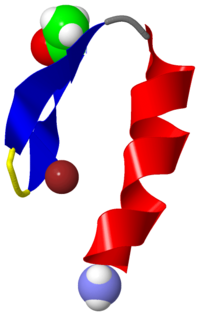
The Zinc Finger
The DNA-binding motif known as the zinc finger was first discovered by Klug in Transcription Factor IIIA in Xenopus laevis, the African clawed toad. TFIIIA is a 344 residue protein that contains 9 repeated modules, which are about 30 residues each, that contain two invariant Cys residues and two invariant His residues[1]. These are able to bind a zinc ion, allowing the protein to fold tightly around it. This protein stabilizer is found in thousands of different proteins in both plants and animals, but not in prokaryotic organisms.
Zinc Finger Structure
The zinc fingers of a protein are normally 20 to 30 amino acids in length and help to create a solid, stable structure [2]. Each finger contains two invariant Cys residues and two His residues and each binds a Zn2+ ion which is liganded tetrahedrally by the Cys and His residues[3]. The zinc finger contains a two-stranded antiparallel beta sheet and an alpha helix.
The three-dimensional solution structure of a zinc finger nucleic acid binding motif has been determined by nuclear magnetic resonance (NMR) spectroscopy. Spectra of a synthetic peptide corresponding to a single zinc finger from the Xenopus protein Xfin yielded distance and dihedral angle constraints that were used to generate structures from distance geometry and restrained molecular dynamics calculations. The zinc finger is an independently folded domain with a compact globular structure in which the zinc atom is bound by two cysteine and two histidine ligands. The polypeptide backbone fold consists of a well-defined helix, starting as alpha and ending as 3(10) helix, packed against two beta strands that are arranged in a hairpin structure. A high density of basic and polar amino acid side chains on the exposed face of the helix are probably involved in DNA binding. [4].
In some zinc finger structures, the His binding residues are replaced by two Cys residues. In other structures, there are six Cys residues that bind two zinc ions. In any case, the Zn2+ ions group together into small globular domains, which eliminates the need for larger, hydrophobic protein cores[5].
Reference
- ↑ Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008
- ↑ Goodsell, David. Zinc Fingers. RCSB. PDB. March, 2007. Web
- ↑ Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008
- ↑ Lee MS, Gippert GP, Soman KV, Case DA, Wright PE. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989 Aug 11;245(4918):635-7. PMID:2503871
- ↑ Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. Hoboken, NJ: Wiley, 2008