</scene>'>
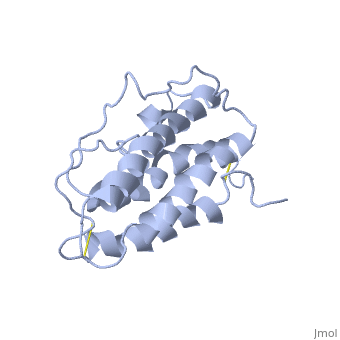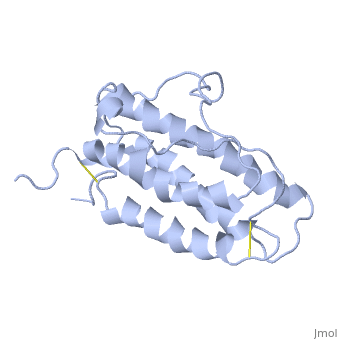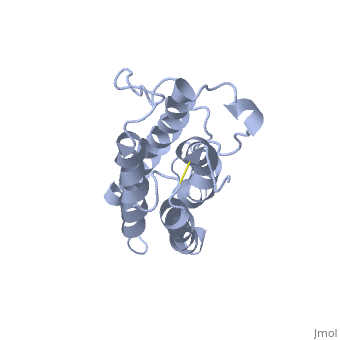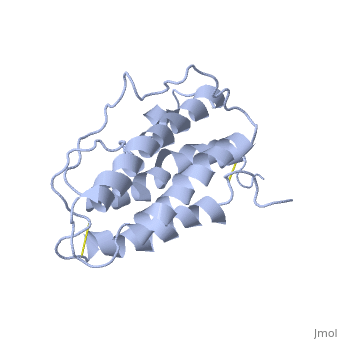
Structure
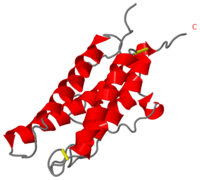
This hormone weight 30.4kD and is constituted to 166 amino acids.
It is composed to 4 alpha-helix: αA, αB, αC and αD which are associated the some in front of the others.
αA et αD are held together by a disulfide bond between and .
αB et αC are held together by a short loop.
The molecule of Erythropoietin have two opposite binding sites with his receptor. The first site include segments of αA, αB and αD and a part of the loop which connects αA and αB. This site include a hydrophobic center which interact with the receptor.
The Phenylalanine 93 of the receptor is critical important for binding of Erythropoietin to his receptor thanks to hydrogen bonds with residues and of Erythropoietin.
In the second interaction site, the Methionine 150 allows van der waals interactions with Arginine 10, the Valine 11 and the Arginine 14 of Erythropoietin.
The hydrophobic interaction include 11 hydrogen bonds between αA and αC of Erythropoietin and his receptor.
Optimal angle for Erythropoietin binding to his receptor.
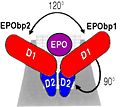
Erythropoeitin with his receptor
Syed and al. in 1998 have showed that Erythropoietin optimal binds at his receptor if only there is an angle of 120° between the two sites of the receptor.
The intracellular surface create by ths angle of 120° allows the optimal induction of Erythropoietin by the intracellulaire way of Kinase.