Introduction
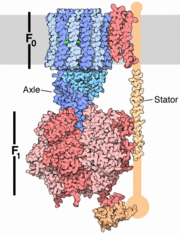
The archaeal A1A0 ATP synthase represent a class of chimeric ATPases/synthase , whose function and general structural design share characteristics both with vacuolar V1V0 ATPases and with F1Fo ATP synthases [3]. A1A0 ATP synthase catalyzes the formation of the energy currency ATP by a membrane-embedded electrically-driven motor. The archaeon in this study,Pyrococcushorikoshii OT3 is an anaerobic thermophile residing in oceanic deep sea vents with an optimal growth temperature of 100 degrees C. Anaerobic fermentation is its principle metabolic pathway.
A hyperthermophilic, anaerobic archaeon was isolated from hydrothermal fluid samples obtained at the Okinawa Trough vents in the NE Pacific Ocean, at a depth of 1395m. The strain is obligately heterotrophic, and utilizes complex proteinaceous media (peptone, tryptone, or yeast extract), or a 21-amino-acid mixture supplemented with vitamins, as growth substrates. Sulfur greatly enhances growth. The cells are irregular cocci with a tuft of flagella, growing optimally at 98 degrees C (maximum growth temperature 102 degrees C), but capable of prolonged survival at 105 degrees C.
[4]
The specific enzymatic process in A-ATP synthase reveals novel, exceptional subunit composition and coupling stoichiometries that may reflect the differences in energy-conserving mechanisms as well as adaptation to temperatures at or above 100 degrees C. Because some archaea are rooted close to the origin in the tree of life, these unusual
mechanisms are considered to have developed very early in the history of life and, therefore, may represent the first energy-conserving mechanisms. [1]
Structure of A-ATP synthase catalytic subunit A
Five steps inside the catalytic A-subunit are critical for catalysis. Substrate entrance, phosphate and nucleotide binding, transition-state formation, ATP formation, and product release. The vanadate bound model mimics the transition state. Orthovandate is a useful transition state analog because it can adapt both tetragonal and trigonal bipyramidal coordination geometry. The Avi structure can be compared to the As sulfate bound structure and the Apnp AMP-PNP bound structure. As is analogous to the phosphate binding (substrate) structure and Apnp is analogous to the ATP binding (product) structure[5].
Within the catalytic A subunit there are the N-terminal domain residues 1-79, 110-116, 189-199, the non-homologous region residues 117-188, the nucleotide binding alpha-beta domain residues 80-99, 200-437, and C-terminal alpha helical bundle residues 438-588 domains. There are 16 helices and 27 strands.
P-Loop
The is the eight residue consensus sequence of amino acid residues 233-241 GPFGSGKT . The P-loop or phosphate binding loop is conserved only within the A subunits and is a loop preceded by a beta sheet and followed by an alpha helix.
K240 and T241 are both contained within the P-Loop and are largely solvent exposed. These residues interact with the phosphate groups of the nucleotide and with a magnesium ion.
Residue is a polar serine molecule that interacts with the nucleotides via a hydrogen bond during catalysis. The distance between residue S238 is longest in As, shortest in Avi and intermediate in Apnp . In As a water molecule bridges the gap, which is removed in Avi. Dehydration of the transition state active site is reversed when ATP forms. In Apnp the water molecule interacts with the y-phosphate of ATP.
Vanadate one occupies the ADP site. Although not at bonding distances the residues P233 G234 L417 stabilize the first vanadate in the transition state with weak nonpoalr interactions. Residues K240 and T241 stabilize with polar interactions.
Transient Binding site
Vanadate two The second is positioned in a region exactly opposite the nucleotide-binding site, where the ATP molecule transiently associates on its way to the final binding pocket in subunit "'B"'. [L417] Is involved in a bifurcated hydrogen bond with the second vandate. This vanadate is also stabilized by weak non polar interactions with P233 F399 F414 A416 and A419, as well as polar interactions with D418 N431 and T434. Similar binding behavior was observed for "'As"' indicating that the substrate molecule has a similar path of entry to the active site in both the "'A"' and '"B"' subunit of the A-ATP synthase and that they have a transient binding position near the P-Loop. It is proposed that Pi binds first to the catalytic site and sterically hinders ATP binding, thereby selectively allowing binding of ADP. The "'Avi"' structure confirms this, since although both ADP and Vi were present in the crystallized solution, the catalytic A-subunit first permits only the binding of the phosphate analogue Vi. Hence the present "Avi"' structure represents a trapped initial transition state showing for the first time both the entering path and the final Vi-bound state in the catalytic subunit.
Comparasons to other known structures
This P-loop has an arched conformation unique to A-ATP Synthase, indicating that the mode of nucleotide binding and the catalytic mechanism is different from that of other syntheses. [6]
For example, in A-ATP Synthases is involved in P-Loop stabilization, but its equivalent (alanine) in subunit B of the F-ATP syntheses subunit beta is a key residue in the catalytic process in moving towards the y-phosphate of ATP during catalysis. By comparing the average distances of the alpha carbons of the P-loop residues to the sulfate, vanadate, and PNP molecules, it was found that the PNP molecule is closest, followed by the vanadate then the sulfate.
In "'F-ATP Synthase"' the homolog to S238 is the non polar A158. Since A158 cannot form hydrogen bonds to interact with the substrate, the P-loop undergoes a conformational change. In A-ATP Synthase the close proximity needed between S238 and the first vandate during transition state is achieved with a hydrogen bond, not a conformational change in the P-loop.
These increased proximities of the catalytically important residues clearly demonstrate that structural rearrangement occurs during catalysis in subunit A.
[7]