ToxT
From Proteopedia
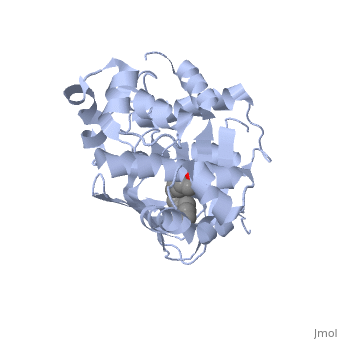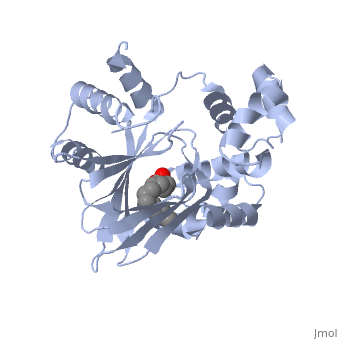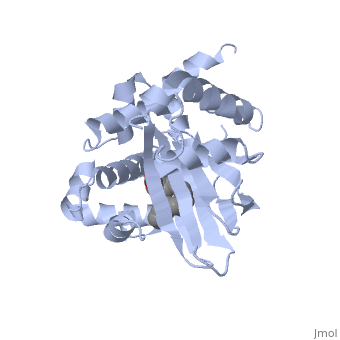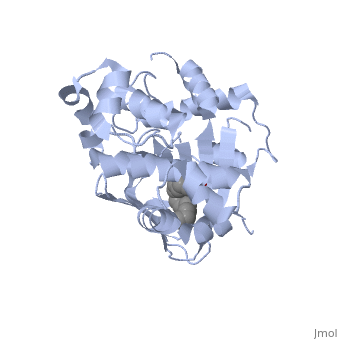
The crystal structure of ToxT is resolved in monomeric form, after isolation from Vibrio cholerae strain O395.[1]
Contents |
Introduction
ToxT is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of Vibrio cholerae[1] and itself. These two factors, cholera toxin (CT)[2] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease cholera[3]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[4]
|
Ligand
In this resolved structure, [5] is present and bound in the beta sheet barrel (further discussed below). This unsaturated fatty acid reduces virulence expression in Vibrio cholerae.
Other Structural Features
ToxT belongs to a family of transcriptional regulators headed by and known as AraC.[1] The AraC family is characterized by a 100 amino acid region of sequence similarity that forms a with two helix-turn-helix motifs (helix in blue, turn in teal). [2] The two HTH regions are linked by a very polar alpha helix (shown in black). The overall domain is composed of seven alpha helices, and is located at the C-terminus.[1]
or "jelly-roll" with three other alpha helices (overall making up the ) contain a . This is largely made up of residues from the N-terminus (Y12, Y20, F22, L25, I27, K31, F33, L61, F69, L71, V81, and V83), and a few from the C-terminus (I226, K230, M259, V261, Y266, and M269). This pocket contains a ligand: , [1] which appears to have a negative effect on virulence when present in vitro. This unsaturated fatty acid, like other UFAs,[6] tend to inhibit genes under the control of ToxT. The
Specifically, the cis-palmitoleate appears to bind directly to ToxT, change its conformation, and thus lower the ability to bind DNA and form dimers.[1]
Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer. The preferred state of ToxT varies between promoters, but binding to the ctx promoter to generate cholera toxin appears to be possible only in the dimer form.[3]ToxT binds to thirteen base pair sequences (can be single, direct, or inverted repeats) called toxboxes in order to activate their respective promoters.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):2860-5. Epub 2010 Feb 1. PMID:20133655
- ↑ Martin RG, Rosner JL. The AraC transcriptional activators. Curr Opin Microbiol. 2001 Apr;4(2):132-7. PMID:11282467
- ↑ Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2372-7. Epub 2007 Feb 5. PMID:17283330 doi:10.1073/pnas.0611643104
Proteopedia Page Contributors and Editors (what is this?)
Ingrid Youngworth, Yang Yang, Michal Harel, Alexander Berchansky, Jaime Prilusky