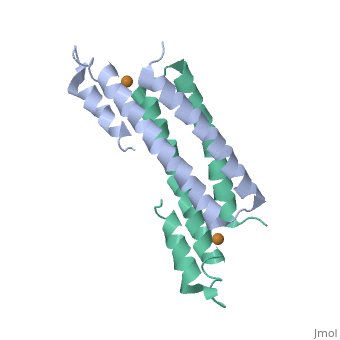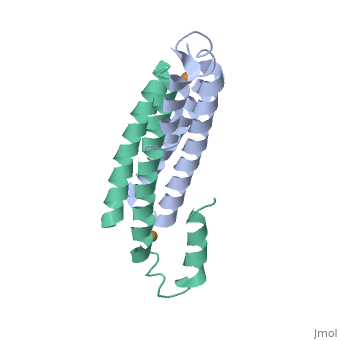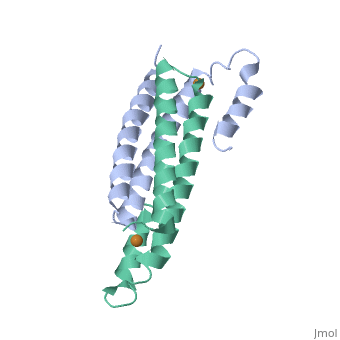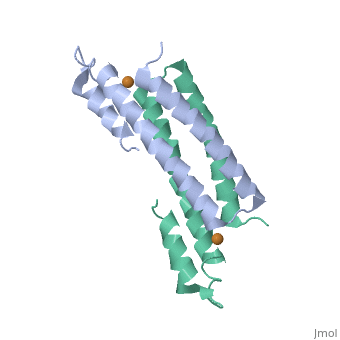
Tetrameric CsoR</scene>binds one Cu(I) per monomer. The protein forms a dimer of dimers with a pore in the tetrameric interface. Each of a dimer unit. Where one monomer is bound to Cu(I) by His61 and Cys65, the other monomer is bound to the metal by Cys35. E. coli RcnR is also tetrameric and has the same protein-to-metal stochiometry.
Research Interests
The mechanism of DNA binding of the CsoR/RcnR family of metal-responsive transcriptional regulators is still unknown. Additionally, RcnR has an added level of complexity because it is reponsive to both Ni(II) and Co(II) binding. The Maroney Lab at the University of Massachusetts Amherst is interested in the conformational changes of RcnR induced by DNA-, Ni(II)-, and Co(II)-binding.