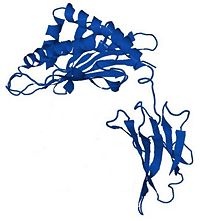
Hemochromatosis protein
From Proteopedia
This sandbox is reserved by Lucie H, ESBS, for a Proteopedia project. Please don't modify anything !
Iron is indispensable for a lot of biological mechanisms in the organism, as a cofactor for many enzymes. However, an overload of this metal is toxic and can trigger serious diseases, such as hemochromatosis. So the iron absorption in the organism is an important mechanism that has to be precisely regulated. The human hemochromatosis protein (HFE) plays a major role in this mechanism.
Contents |
Description of HFE structure
|
HFE is a transmembrane glycoprotein composed by 343 amino acids. The gene coding for this protein is localized on the short arm of the 6th chromosome.
HFE is a MHC-class-1-like protein. In fact, it's composed by three extracellular domain : and , a transmembrane domain and a short cytoplasmic domain. [1] The α1 and the α2 domains form a platform composed of eight topped by . [2] Moreover, as every MHC class 1 protein, it can bind to a microglobulin : . This interaction is indispensable for HFE activity.
|
HFE has a 2 identified interactors. It can bind Transferrin Receptor 1 (TfR1) through its α1 and α2 domains. TfR1 is an homodimer, so one homodimer can bind two HFE molecules (in the animation, the repeated part of the complex formed is represented in transparency). The core of the interface is formed by the α1 helix from HFE and the helices 1 and 3 from the (the helical domain is the domain involved in TfR dimerization, residues 607-760). This interface contains a lot of hydrophobic residues. For example, . [3]
HFE can also bind Transferrin Receptor 2 (TfR2) through its α3 domain. Transferrin is an iron carrier. To understand better the mechanism in which HFE is implicated, we also have to know that on TfR1, the binding sites of HFE and Holo-Tf (that's to say transferrin without iron) are overlaping. [1]
Biological process of iron metabolism
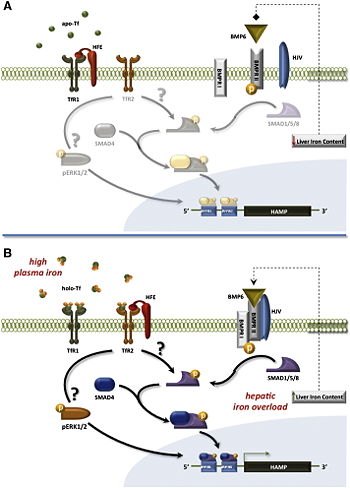
Iron homeostasis is regulated by a closed loop. The main part of organic iron is localized in the erythorcytes and in the bone marrow. Macrophages recycle iron from senescent erythrocytes so that it can be used once again. The liver acts as a buffer and stores the surplus iron. But this loop has some leaks through bleeding or sweating. That's why this circuit needs an external contribution provided by food. [4]
Iron provided by alimentation is absorbed by the enterocytes of the small instestin, which are the unique frontdoor. Once absorbed by the divalent-metal transporter 1 (DMT1), it can either be stored by the ferritin or be distributed in the organism thanks to different factors according the organism's needs. Ferrous ions are released from the epithelial cells through iron-regulated protein 1 (IREG1), also known as ferroportin. Back to their ferric state, they are able to bind transferrin (Tf) and to be transported across the vascular endothelium into the blood. [5]
Cells which need iron for their metabolism are presenting at their surface the transmembrane proteins TfR1 and TfR2 as well as HFE/β2-microglobulin complex. As apo-Tf doesn't have any affinity for TfR1, it allows the formation of HFE/β2-microglobulin/TfR1 complex. This complex prevents the interaction between HFE/β2-microglobulin and TfR2, interaction needed to stimulate hepcidin synthesis. Hepcidin is an hormon that degrades ferroportin, preventing iron liberation by enterocytes and macrophages.
When there is no iron in the organism, there is a low hepcidin rate that enable iron liberation. Iron binds to transferrin, forming the holo-Tf. This complex, having a higher affinity for TfR1 than TfR2, can bind TfR1 to be endocyted by user cells. HFE/ β2-microglobulin is then going to bind to TfR2. This interaction activates the pathway BMP6-SMAD, which stimulates hepcidin synthesis. Then, hepcidin inhibits iron liberation in the organism. Iron homeostasis is maintained. [4]
Hemochromatosis disease
|
Hereditary hemochromatosis (HH) is a disease that can lead to a iron overload of many organs, the liver in particular. Thus, it can cause hepatic cirrhosis, hepatocellular carcinoma, cardiomyopathy, and so on ... The most common form of HH is due to mutations in the protein HFE.
Most patients suffering from hereditary hemochromatosis are homozygous for a the single mutation Cys260Tyr. This substitution implicates the , which is located in the α3 domain and which is forming a disulfide bound with the cystein 203 (yellow). By breaking this disulfide bound, this mutation creates a misfolded HFE that cannot interact with β2-microglobuline. This mutation also prevents cell-surface expression.
In some hereditary hemochromatosis cases, a second mutation in the α1 domain is implicated : His41Asp. is located in a loop within the α1 domain, and usually interacts with Asp 73 (yellow) to form a salt bridge. The substitution may cause a local rearrangement of this loop to avoid juxtaposition of two negative charges. In this state, HFE cannot fully assure its function.
When HFE is inactive, it cannot activate hepcidin synthesis, and the macrophages and enterocytes are liberating all the iron available into the blood.[2]
References
- ↑ 1.0 1.1 Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009 Mar;9(3):217-27. PMID:19254567 doi:10.1016/j.cmet.2009.01.010
- ↑ 2.0 2.1 Lebron JA, Bennett MJ, Vaughn DE, Chirino AJ, Snow PM, Mintier GA, Feder JN, Bjorkman PJ. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998 Apr 3;93(1):111-23. PMID:9546397
- ↑ Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000 Jan 6;403(6765):46-53. PMID:10638746 doi:10.1038/47417
- ↑ 4.0 4.1 4.2 Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta. 2011 Nov 4. PMID:22085723 doi:10.1016/j.bbagen.2011.10.013
- ↑ Chorney MJ, Yoshida Y, Meyer PN, Yoshida M, Gerhard GS. The enigmatic role of the hemochromatosis protein (HFE) in iron absorption. Trends Mol Med. 2003 Mar;9(3):118-25. PMID:12657433
Proteopedia Page Contributors and Editors (what is this?)
Lucie Hartmann, Michal Harel, Wayne Decatur, Jaime Prilusky, Nathalie Faggianelli