Sandbox 22
From Proteopedia
This sandbox is currently being used to develop a page for papain. Please do not edit it. Thanks, Samuel Bray
Contents |
Papain
Exciting Introduction!
Papain. Meat tenderizer. Old time home remedy for insect, jellyfish, and stingray stings[1]. Who would have thought that a sulfhydryl protease from the latex of the papaya fruit, Carica papaya and Vasconcellea cundinamarcensis would have such a practical application beyond proteopedia?
Introduction
General Characteristics
Papain is a 23.4 kDa, 212 residue cysteine protease, also known as papaya proteinase I, from the peptidase C1 family (E.C. 3.4.22.2).[2][3] It is the natural product of the Papaya(Carica papaya)[4], and may be extracted from the plant's latex, leaves and roots.[5] Papain displays a broad range of functions, acting as an endopeptidase, amidase, and esterase,[6] with its optimal activity values for pH lying between 6.0 and 7.0, and its optimal temperature for activity is 65 °C. Its pI values are 8.75 and 9.55, and it is best visualized at a wavelength of 278 nm.[7]
Common Uses
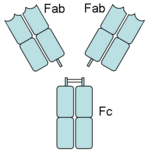
Papain digests most proteins, often more extensively than pancreatic proteases. It has a very broad specificity and is known to cleave peptide bonds of basic amino acids and leucine and glycine residues, but prefers amino acids with large hydrophobic side chains. This non-specific nature of papain's hydrolase activity has led to its use in many and varied commercial products. It is often used as a meat tenderizer because it can hydrolyze the peptide bonds of collagen, elastin, and actomyosin. It is also used in contact lens solution to remove protein deposits on the lenses and marketed as a digestive supplement. Another place papain has found use is clinical medicine, where it is used to treat pain, swelling, and fluid retention following trauma and surgery. [8] Finally, papain has several notable uses in general biomedical research, including a gentle cell isolation agent, production of glycopeptides from purified proteoglycans, and solubilization of integral membrane proteins. It is also notable for its ability to specifically cleave IgG and IgM antibodies above and below the disulfide bonds that join the heavy chains and that is found between the light chain and heavy chain. This generates two monovalent Fab segments, that each have a single antibody binding sites, and an intact Fc fragment, as shown in the image to the right:[6]
History
Papain's enzymatic use was first discovered in 1873 by G.C. Roy who published his results in the Calcutta Medical Journal in the article, "The Solvent Action of Papaya Juice on Nitrogenous Articles of Food." In 1879, papain was named officially by Wurtz and Bouchut, who managed to partially purify the product from the sap of papaya. It wasn't until the mid-twentieth century that the complete purification and isolation of papain was achieved. In 1968, Drenth et al. determined the structure of papain by x-ray crystallography, making it the second enzyme whose structure was successfully determined by x-ray crystallography. Additionally, papain was the first cysteine protease to have its structure identified.[6] In 1984, Kamphuis et al. determined the geometry of the active site, and the three-dimensional structure was visualized to a 1.65 Angstrom solution.[9] Today, studies continue on the stability of papain, involving changes in environmental conditions as well as testing of inhibitors such as phenylmethanesulfonylfluoride (PMSF), TLCK, TPCK, aplh2-macroglobulin, heavy metals, AEBSF, antipain, cystatin, E-64, leupeptin, sulfhydryl binding agents, carbonyl reagents, and alkylating agents.[6]
Structure
|
General Structural Features
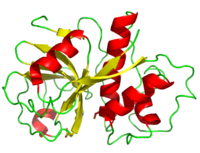
Papain is a relatively simple enzyme, consisting of a single 212 residue chain. Its secondary structure is composed of 21% (45 residues comprising 7 sheets) and 25% (51 residues comprising 7 helices). The rest of the residues, accounting for over 50% of the enzymes structure, make up ordered non-repetative sequences.[10] These secondary structures may be traced from the N- to C-terminus by means of . As shown in this scene, the red end begins the protein at the N-terminus, and can be traced through the colors of the rainbow to the blue end at the C-terminus.
A majority of papain's residues are As with all proteins, folding to form secondary and tertiary structures is largely a product of the aggregation of hydrophobic residues as they are excluded by water (hydrophobic residues shown in purple). A majority of these hydrophobic residues form hydrophobic cores within each of the lobes ensuring their stability (surface residues are transparent, buried hydrophobic residues are opaque and colored).Seeing its further shows remaining mostly on the exterior while sequestering near the center. Observations have revealed that the proteins atomic positions are more ordered going from outside toward the center and also disclose the hydrophobic core of the enzyme.[11]