We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jamie Abbott/Sandbox2
From Proteopedia
Contents |
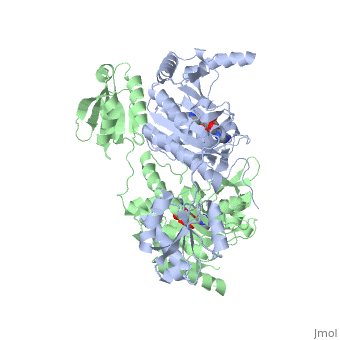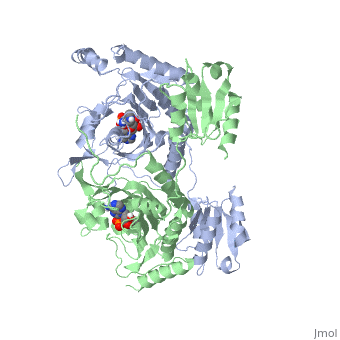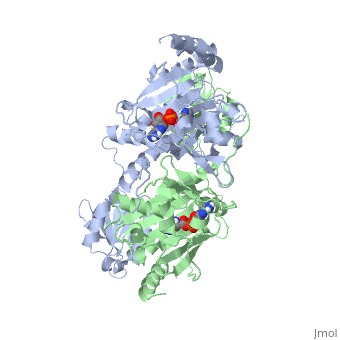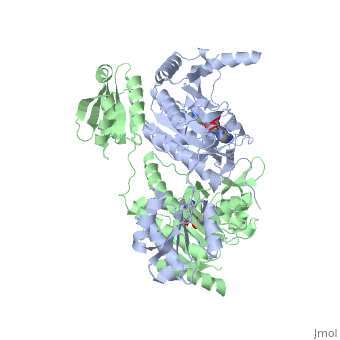
Histidyl-tRNA Synthetase
Histidyl tRNA Synthetase (HisRS) is a 94kD homodimer that belongs to the Class II of aminoacyl-tRNA synthetases (aaRS). Class II enzymes have a composed of anti-parallel β-sheets and α-helices (residues 1-325).
Function and Catalysis
| |||||||||||
Mechanism
3D Structures of Histidyl-tRNA Synthetase
References
- ↑ aaRSbook