This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Jamie Abbott/Sandbox2
From Proteopedia
Contents |
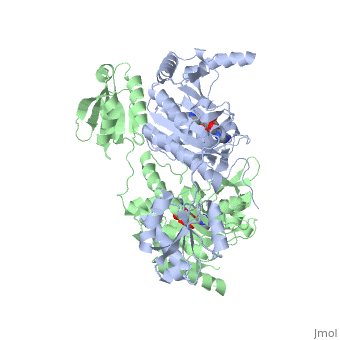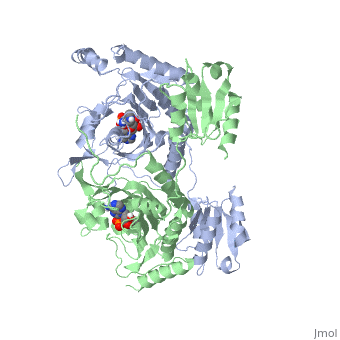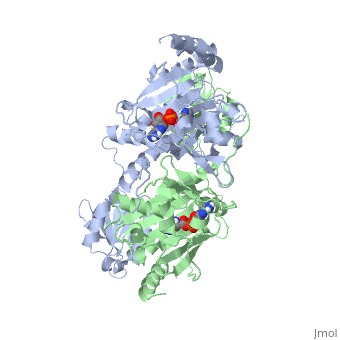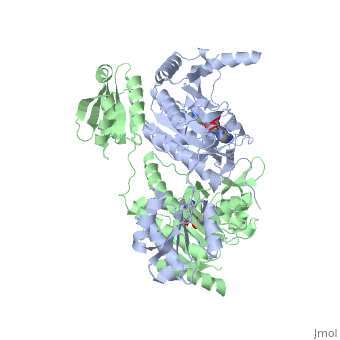
Histidyl-tRNA Synthetase
Histidyl tRNA Synthetase (HisRS) is a 94kD that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). Aminoacyl-tRNA synthetases have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons[1]. Class I and Class II differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA[1]. Class II enzymes have a composed of anti-parallel β-sheets and α-helices (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be anticodon binding domain); class IIb, whose anticodon binding domain is located on the N-terminal side of the fold; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases.[2]
Function and Catalysis
| |||||||||||
Mechanism
Evolutionary Conservation
Structural Homology
3D Structures of Histidyl-tRNA Synthetase
References
- ↑ 1.0 1.1 Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203-6. PMID:2203971 doi:http://dx.doi.org/10.1038/347203a0
- ↑ Cusack S, Hartlein M, Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489-98. PMID:1852601
- ↑ 3.0 3.1 Francklyn, C., and Arnez, J.G. (2004) in Aminoacyl-tRNA Synthetases (Ibba, M.,Francklyn, C.,Cusack, S.. Eds.) Landes Publishing, Austin, TX