User:Jamie Abbott/Sandbox2
From Proteopedia
Histidyl-tRNA Synthetase
Histidyl tRNA Synthetase (HisRS) is a 94kD that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). Aminoacyl-tRNA synthetases are classified as ligases where as they require a high energy ATP cofactor to ligate a specific amino acid to their cognate tRNAs. Histidyl-tRNA synthetase more specifically attaches amino acid histidine to tRNAHis. Histidine is a unique amino acid often part of enzyme active sites where it can act as either a base or an acid during acid-base catalysis. Aminoacyl-tRNA synthetases have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons[1]. Class I and Class II differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA[1]. Class II enzymes have a composed of anti-parallel β-sheets and α-helices (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be ); class IIb, whose anticodon binding domain is located on the N-terminal side of the fold; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases.[2]
Substrate Specificity
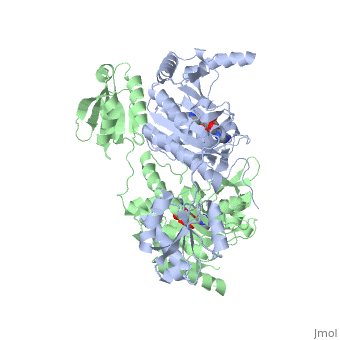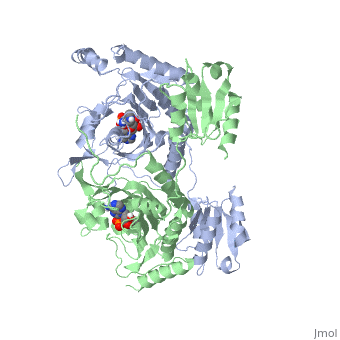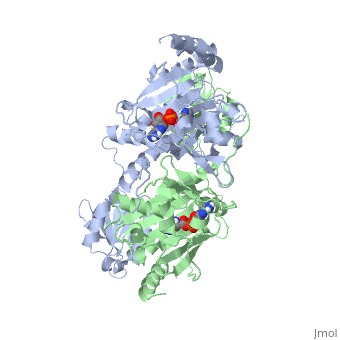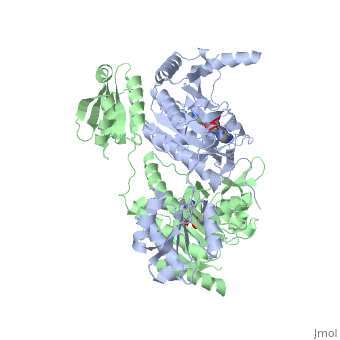
The first crystal structure solved for histidyl tRNA-synthetase was of e.coli. To date structures of e.coli HisRS complexed with ATP, AMP, histidine, competitive inhibitor (histidinol), histidyl-adenylate, and 5’-O-[(L-Histidnylamino)sulfonyl] adenosine have been extensively explored. Histidine Binding Pocket The active site to HisRS contains a composed of highly conserved found in distinct sequences motifs. First, the LV/AAGGGLDYY loop (or ) forms one wall of the binding pocket. This HisA loop is highly conserved and extends over a part of the active site[3]. Second, the glycine-rich β-strand (sequence AGGRYDGL preceding ) comprises the histidine binding pocket floor and wall. Finally, conserved side chains that make direct contact with histidine are Glu83 and Gly127 (), which contact the α-amino and α-carbonyl functional groups, respectively, and Glu131 (motif II) and Tyr264, which make hydrogen bonds to the Nδ and Nε, respectively, of the imidazole ring[3].
ATP Cofactor Binding Many interactions are required to prepare adenosine triphosphate (ATP) for attack by a bound histidine molecule and encourage the magnesium pyrophosphate moiety to act as a leaving group. Residues in the β strands and the loop portion of motif 2 are important in ATP contacts for HisRS[4]. Generally, residues involved in are among the most highly conserved in the HisRS family and for the most part shared by all members in class II. The π-stacking interaction between the adenine ring of ATP and provides specificity in the binding of ATP. The recognition of the N6 amino group of ATP involves the main chain carbonyl of Tyr122. The ATP ribose 2’ OH forms an additional contact with HisRS by hydrogen bonding with the main chain carbonyl of Thr281. There are four arginine residues that mediate interactions with the triphosphate group of ATP. First, the conserved Arg113 forms a bridging interaction with the α and β phosphates of ATP in the crystal structure of e.coli HisRS complexed with histidinol [4]. Also, the γ phosphate forms salt bridges with conserved Arg121 and Arg311 in a complex with ATP. However, when HisRS is complexed with the adenylate, the Arg121 and Arg311 interactions are absent and adopt different conformations[5][4]. Furthermore, Glu115 also assists in the stabilization the triphosphate group of ATP in a position such that it points back towards the adenine base. This conformation of ATP is evidently unique to class II aaRS[6]. The α phosphate of ATP interacts with conserved residue Arg113. The β and γ phosphates are neutralized by two coordinated magnesium ions that are positioned by water molecules and conserved Glu115[3]. Also, the γ phosphate forms additional interactions with conserved Arg121 and Arg311.
A Role for Coordinated Metal Ions The β and γ phosphates are neutralized by two coordinated magnesium ions that are positioned by water molecules and conserved Glu115. Weak electron density, consistent with a bound Mg2+ ion, was observed in an electron density map for the HisRS:histidinol:ATP complex [4]. This particular Mg2+ ion coordinates the β and γ phosphates of ATP. Arnez et al. further defined the locations of magnesium ions by taking a crystal of HisRS:histidinol:ATP complex and soaked it in manganese(II) chloride MnCl2. Data collected and analyzed by Arnez et al. showed that two Mn2+ ions coordinate the β and γ phosphates of ATP. Furthermore, interatomic distances between the Mn2+ principal ion and the β phosphate oxygen is approximately 0.5 Å, which would be expected to contribute to catalysis by weakening the bond between the α and β phosphates of ATP. In similar manganese soaking experiments with another classIIa aaRS, SerRS, positions of the principal metal ion was mapped to coordinate the α and β phosphates[7]. The functional role for the metal ion coordination between the α and β phosphates for SerRS is a metal-catalyzed mechanism for the adenylation reaction. Interestingly, Arg259 in the HisRS:ATP complex resides the position occupied by the metal catalyst Mg2+, in classIIa SerRS. Arg259 and Arg113 serving in place of a Mg2+ ion is unique to HisRS compared to other classII aaRS. In other classII aaRS enzymes have conserved carboxylate groups to assist in coordination of metal ions to carryout catalysis, while HisRS has in place residues Glu270 and Thr281 that have poor geometry for metal coordination but participate in the arginine salt bridge switch[4]. | |||||||||||
Mechanism
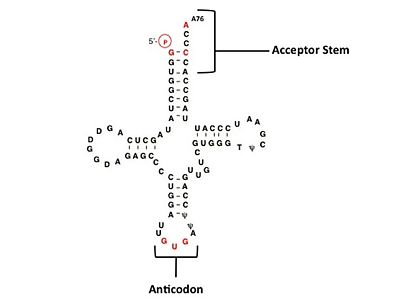
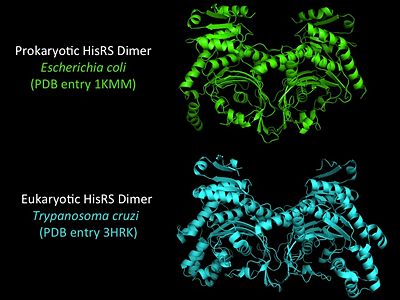
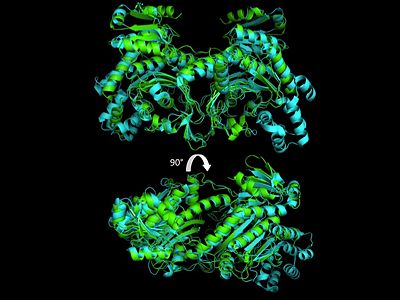
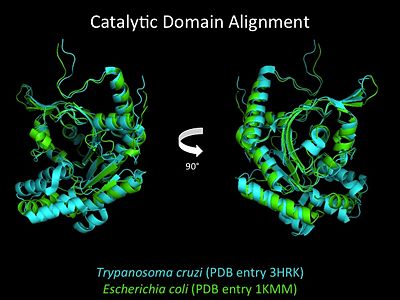
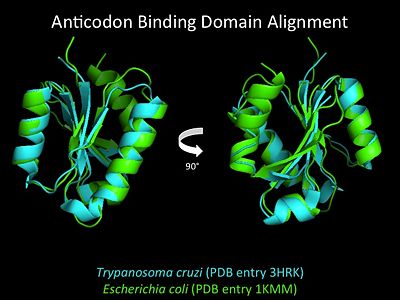
Electrophilic CatalysisThe HisRS active site contains a highly conserved residue in the HisRS family, Arg259, that takes part in electrophilic catalysis for the adenylation reaction. This active site arginine residue is not present in other aaRS class II enzymes. As noted in the above discussion about metal ion coordination Arg259 and Arg113 are positioned to interact with the α-phosphate of ATP to assist in the adenylation reaction. First, as Arg259 is positioned on the HisA loop serves to fix the α-carboxylate group of the histidine substrate as the [8]. Second, one ηN of the guanidinium group of Arg259 is positioned approximately 3Å from the α-phosphate of ATP while the other ηN hydrogen bonds with phenolic group of Tyr264. Furthermore, the Tyr264 residue is then stabilized to also hydrogen bond to the Nδ of the histidine substrate[3]. A comparison of Glu270 in the HisRS:histidinol and the HisRS:adenylate complexes provides further structural information into how Arg259 may serve a role in catalysis. In the HisRS:histidinol complex a water-mediated interaction between Glu270 and εN of Arg259. However, in the HisRS:adenylate complex Glu270 moves to form a salt bridge with the guanidinium group and to exclude the water molecule. This movement serves as a salt bridge switch that may weaken the ionic interaction between Arg259 and the α-phosphate(arnez1st step) and stabilize adenylate formation in the active site. Also, Arg113 as well as Arg259 are arranged to interact with of ATP and and also stabilize negative charge developed on the non-bridging oxygens α-phosphate during the transition state [3]. Evidence for Arg259 playing a critical role in catalysis is further supported by mutational studies where a two or three log decrease in activity is observed when Arg259 is substituted with a histidine [4] or other amino acids[9]. Utilizing Arg259 for catalysis is unique to HisRS as other class II aaRS enzymes, AspRS[10] and SerRS[7], use a divalent magnesium metal ion to coordinate the α-phosphate of ATP and serve as an electrophilic catalyst. Substrate Assisted Catalysis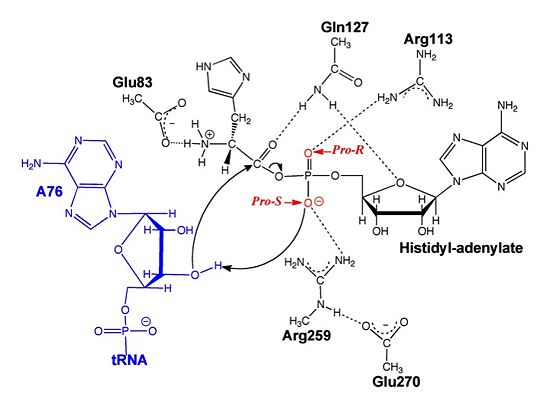 Substrate assisted mechanism catalyzed by histidyl-tRNA synthetase purposed by Francklyn et al.[11] The second reaction carried out by HisRS, aminoacylation, requires the decomposition of a mixed anhydride (the ) to form an aminoacyl ester on the 3’OH of tRNAHis. It was initially hypothesized that Glu83, acting as a general base, would improve the rate of this reaction. However, while Glu83 is in a favorable position in the active site to function as a base it is also situated to neutralize the α-amino group of the histidine substrate. Thus, mutational analysis of Glu83[11] suggests that it does not act as a base but forms a salt bridge with the α-amino group of histidine, neutralizing it’s charge, and satisfying a critical electrostatic interaction. The mechanism for transfer of the aminoacyl moiety from the aminoacyl-adenylate, generated in the adenylation reaction, onto it’s cognate tRNA is most easily explained by substrate assisted catalysis (SAC). Substrate assisted catalysis preformed by HisRS is described as the occurrence of bond formation between the 3’OH of tRNAHis and the α-carboxylate carbon of the aminoacyl adenylate prior to the cleavage of the bond joining the α-carboxylate carbon to the axial oxygen of the α-phosphate. This concerted SAC mechanism, rationalizes the considerable pKa difference between the 3’OH of tRNA (pKa =16) and the nonbridging Sp oxygen (pKa= -1)[11]. Therefore, as the bond between the tRNAHis nucleophile and the α-carboxylate is formed, the pKa for the 3’OH would be expected to drop sharply and the pKa of the nonbridging oxygen would tend to rise as the bond to the α-carboxylate lengthens and breaks[11]. Histidine tRNA Recognition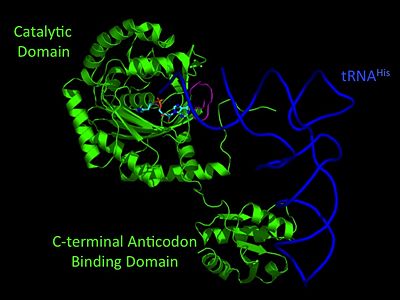 Model of HisRS-tRNAHis Complex predicted by homology modeling with AspRS-tRNAAsp crystal structure[12] The accuracy of protein synthesis is dependent upon the ability of aminoacyl-tRNA synthetases to specifically recognize cognate tRNA and attach the appropriate amino acid. The identity nucleotides that define tRNA isoacceptor systems are primarily concentrated in the anticodons and acceptor stems of tRNAs, providing functional groups that can be accessed by specificity-determining side chains on the enzymes [13],[14]. Although, tRNA identity can also emerge from the presence of modified bases[15]. Key identity elements on E. coli histidine tRNA include the 5’ phosphate, G-1:C73 base pair in the acceptor stem and the GUG anticodon. Mutations of these identity elements diminishes aminoacylation in vitro[16] [17] [18]. Residues throught to be involved in the recognition of these identity elements include; Arg123, Arg116, and Gln118. Substitution in Arg123 as a putative contact to the 5’phosphate, produced a 200 fold decrease in aminoacyl-transfer[19]. Similar kinetic defects in aminoacyl-transfer were also observed for Arg116 and Gln118 [19]. Evolutionary ConservationStructural HomologyDuring the course of evolution the catalytic domain has been the most preserved between prokaryotic and eukaryotic HisRS enzymes. Overall the sequence homology shared between prokaryotic, e.coli, HisRS and eukaryotic, s.cerevisiae, HisRS is only 28.5% in the catalytic domain, encompassing 235 residues ARNEZ97. The eukaryotic HisRS enzymes share much greater overall sequence homology, for example s.cerevisiae and h.sapiens HisRS have 43.5% shared sequence homology. Both eukaryotic and prokaryotic HisRS sequences share a conserved GExExxxG motif in the C-terminal domain. However, overall sequence homology shared between eukaryotic and prokaryotic C-terminal anticodon binding domain is minimal. More recently a crystal structure for eukaryotic Trypanosomal brucei and Trypanosomal cruzi histidyl-tRNA synthetase was solved[20]. While both the human and trypanosomal HisRS sequences are part of the same eukaryotic branch on the HisRS phylogenetic tree[21] there is less than 30% sequence identity shared between them. Also, the sequence identity between trypanosoaml HisRS and bacterial HisRS is less than 30% [22]. Furthermore, many higher eukaryotes, more specifically mammals, have separate cytosolic and mitochondrial HisRS enzymes. The human HisRS genes, which arose from inverted gene duplication[23][24], HARS and HARS2 encode for the cytosolic and mitochondrial HisRS enzymes respectively. The gene products of HARS and HARS2 share approximately 79% sequence identity. 3D Structures of Histidyl-tRNA SynthetaseBacteria Eukaryota Archara References
| ||||||||||||||||