Sandbox Reserved 480
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Phenylalanine Hydroxylase
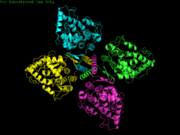
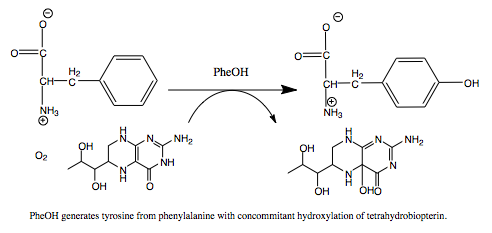
Phenylalanine Hydroxylase (PheOH) is a type of enzyme involved in the catalization of phenylalanine into tyrosine. It belongs to the family of aromatic amino acid hydroxylases. Some other enzymes in this family include tyrosine hydroxylase and tryptophan hydroxylase. These enzymes require oxygen and tetrahydrobiopterin to convert their amino acid substrates into products. StructureThe regulatory domain affects the function of the active site. When the regulatory domain is , the active site is closed off and prevented form interacting with the substrates. In this scene the chain is colored from N-terminus to C-terminus blue to red and the active site and Fe (III) is colored black. In the PheOH, the active site is open to the subtrates and can convert them to products. PheOH is a tetrameric enzyme, consisting of 2 asymmetric components. It contains an It has been observed as a and tetrameric structure. This is the site within the subunit. This includes the residues His, His, and This enzyme has two , Fe (III) and . PhenylketonuriaPhenylalanine hydroxylase is the rate limiting exzyme invovled in the metabolism of phenylalanine. With Fe (III), oxygen, and tetrahydrobiopterin, PheOH hydroxylates Phe into Tyr. Tyr goes on to make L-dopa and eventually dopamine. MechanismReferences |