This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 451
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Kinesin
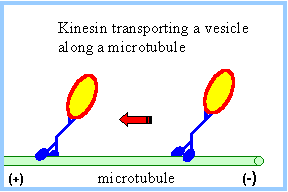
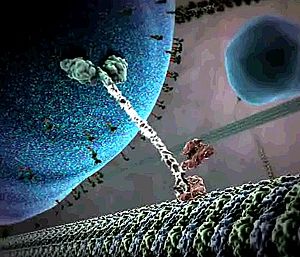
IntroductionKinesins are a class of motor proteins found in eukaryotic cells which are capable of converting chemical energy to mechanical work by hydrolyzing ATP. These motor proteins are essential in transporting molecules that are incapable of reaching their proper destinations by passive methods (e.g. diffusion) but require more active methods of transport like the use of molecular motors. Kinesins are composed of a motor domain, which binds ATP at the and hydrolyzes it to ADP converting that chemical energy into motion, and a cargo-binding domain which holds the species being transported. A long flexible stem connects the cargo-binding domain to the motor domain. Most Kinesins transport large cargo like lysosomes from the nucleus to the cell membrane using the array of microtubles found in the cell in a method known as anterograde transport. Motor proteins that carry molecules from the membrane of the cell to the center are called Dyneins StructureKinesin is a dimer composed of two identical subunits. Each subunit is comprised of two domains. At the amino terminal end is the motor domain which is responsible for motility and at the carboxy terminal end is the cargo-binding domain which is responsible for the attachment of cargo for transport. These two domains are connected by two alpha helical coiled coils called the stalk . The stalks of the two motor domains intertwine to direct dimerization of the two motor domains. Dimerization of the coiled coils are stabilized by the repeating pattern of both the and the of the amino acids, known as a heptad repeat.The motor domain is comprised of approximately 320 highly conserved amino acids and each of its identical subunits consists of an extended between . The structure of many Kinesin proteins have been solved using X-ray crystallography. Mechanism of ActionEach motor domain head contains an active site (where ATP binds) and a binding site for the microtuble binding. The ATP binding site binds ATP and very quickly hydrolyzes the high energy phosphate bond to produce ADP. The protein structure shown here has bound rather than ATP. There are two proposed mechanisms of movement for the kinesin protein, hand over hand and inchworm. In the hand over hand mechanism, each motor domain head alternates leading and trailing roles with each step. In this mechanism when ATP binds one head, the phosphate bond is quickly hydrolyzed to ADP-Pi providing enough energy for that head to tilt forward and the same happens to the other head in a sequential manner resembling a "walking" motion. In the inch worm mechanism, only one head tilts forward and the other catches up to it thus resembling an inchworm. Of the two proposed methods of movement, the hand over hand mechanism (shown below) is more widely accepted. Shown here is an animated still of the Kinesin protein carrying its cargo along the microtuble. Note the size of the cargo in comparison to the size of the proteins. Kinesins are able to transport cargo much larger than their own size due to their flexible but rugged structure. Medical ImplicationsThe use of Kinesins is essential to cell function in that it helps transport large molecules like lysosomes from the nucleus of the cell to the cell membrane. Unfortunately for the cell, many viruses like the HIV or herpes virus are able to hijack this system of transport in order to help facilitate viral replication. The HIV virus hijacks the Kinesin protein and uses it to transport newly assembled viral bodies to the membrane of the cell where it has the opportunity to spread to other cells. Studies have shown that drugs that disrupt microtuble formation have also disrupted viral replication. Ultimately, the study of Kinesins helps shed light on microtuble-based motility and spread of viruses. References1. "Kinesin." Interactive Concepts in Biochemistry. Web. 1 May 2012. <http://higheredbcs.wiley.com/legacy/college/boyer/0471661791/structure/kinesin/kinesin.htm>. 2. Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Kinesin, Dynein, and Intracellular Transport. 3. Goodsell, David. "Kinesin." RCSB PDB-101. Web. 01 May 2012. <http://www.rcsb.org/pdb/101/motm.do?momID=64>. 4. Kinesin Moves by an Asymmetric Hand-Over-Hand Mechanism Charles L. Asbury, Adrian N. Fehr, and Steven M. Block Science 19 December 2003: 2130-2134.Published online 4 December 2003 [DOI:10.1126/science.1092985] 5. "Microtubule Motors." Rensselaer Polytechnic Institute (RPI). Web. 01 May 2012. <http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/kinesin.htm>. 6. Leibier, John, Mike Astrachan, and David Bolinsky. "The Inner Life of the Cell « XVIVO." The Inner Life of the Cell « XVIVO. Web. 30 Apr. 2012. <http://www.xvivo.net/the-inner-life-of-the-cell/>. 7. Dodding, Mark P., and Michael Way. "Coupling Viruses to Dynein and Kinesin-1." Nature.com. Nature Publishing Group. Web. 02 May 2012. <http://www.nature.com/emboj/journal/v30/n17/full/emboj2011283a.html>. |