User:Susan Corradino
From Proteopedia
|
- Graduate student
- State University of New York, Old Westbury
- Old Westbury, NY, USA
- Education (Biology). B.S. Marine Vertebrate Biology.
Contents |
Introduction
Calnexin is an integral protein found in the plasma membrane of the endoplasmic reticulum. This protein functions as a chaperone protein , forming a super-complex which aids in the formation of the MHC complex. The MHC complex comes in two forms, MHC class I and MHC class II. This molecule is responsible for peptide presentation to T cells in the adaptive immune response. MHC I complex forms and matures in the endoplasmic reticulum of the cell. Once the peptide is bound to the heavy chain of the peptide binding cleft, the molecule will migrate out of the endoplasmic reticulum to the cytosol eventually making its way to the cell surface for presentation to T lymphocytes. T cells will bind to the MHC I complex when antigen recognition has occurred (Janeway 2001). Without calnexin, the MHC complex would be compromised and ultimately be unable to present the peptide required for the immune response. An interesting additional function of the protein is to ensure that misfolded glycoproteins remain in the ER until they are disposed of by the appropriate regulating structures. Calnexin has also been described as a player in aiding in the proper folding of polypeptides.
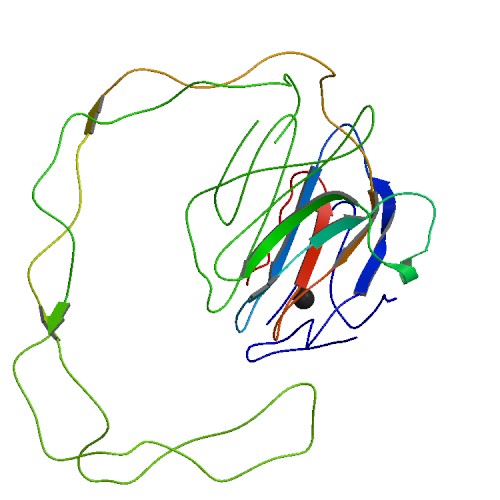
3D Structure
This molecule is asymmetrical in shape with two unique domains. The long protruding arm of the protein is thought to function with the communication between calnexin and other proteins, specifically ERp57, a secondary chaperon protein in the formation of the MHC I complex, this section of the protein gives the protein a unique crystal structure that is unlike other proteins (Schrag et al 2001). This arm also provides the contact point for all intermolecular communication (Schrag et al 2001). There are two significant disulfide bonds found in the long arm of calnexin, - and - (Schrag et al 2001). Important amino acids in this section of the crystal structure of calnexin include and residues of found in the lectin domain as well as and residues . This aspect of the crystal structure will allow the arm of the molecule to wrap around associate proteins to form the super complex for the MHC I (Schrag et al 2001).The second domain which is globular lectin in nature provides the ability to monovalently bind glycan.
Function
Calnexin is a chaperone protein that aids in the folding of the MHC I complex. Research shows that calnexin assists in the folding of the heavy chains of the molecule (Vassilakos et. al 1996). Research shows in mice, D. melanogaster, and humans, that when calnexin was experimentally removed, the heavy chains were unable to fold properly resulting in the misfolding and inhibition of complex formation (Vassilakos et. al 1996).
Calnexin has an additional function when paired with [http://en.wikipedia.org/wiki/Glucosidases glucosidases), the molecule will help to regulate the folding of glycoproteins. In the absence of calnexin glycans will fold as a drastically lower rate (Molinari et al 2000).
Calnexin has been conservered through out evolution as it is a molecule found in most eukaryotic cells.
Future Research
Journal article searched did not yield results that indicate studies performed or research on the ability of pathogens to mimic the structure of the critical protein. It is reasonable to say that a virus would develop evolutionary adaptations that would allow it to use mimicry to prevent the formation of the MHC I complex which would in turn prevent peptide presentation.