User:Elizabeth Tomao/sandbox1 alphaB-crystallin
From Proteopedia
|
The Mysterious Roles of αβ-crystallin
Introduction
The protein αβ-crystallin is small heat shock protein (HSP) that is part of crystallin family as well as the α cystistallin subfamily. It is closely related to αA- crystalin, there are a few major differences between the two proteins. αA crystallin is mostly found in the ocular lens, whereas αβ-crystallin can be found in the ocular lens but also in other tissues such as the lungs, kidneys, brain, and cardiac muscles. Heat shock proteins may also be known as stress proteins because they are activated under conditions of stress; such as heat, change in pH, amount of oxygen and other materials such as metals. When activated these heat shock proteins act as chaperones, by assisting in the folding and folding and to prevent the aggregation or misfolding of other proteins. αβ-crystallin accumulates in tissues when they are exposed to any type stress, therefore it is common in a tissue that is being affected by a disease. It has been found in the following tissues brain, heart, kidneys, skeletal muscle, placenta, and skeletal muscle and associated with such diseases as Parkinson ’s disease, Alzheimer’s Disease, Alexander’s Disease, Multiple Sclerosis, Cancers, and Heart Disease. This small protein acts as a form of defense by preventing the disease from attacking certain cells.
Structure and Function
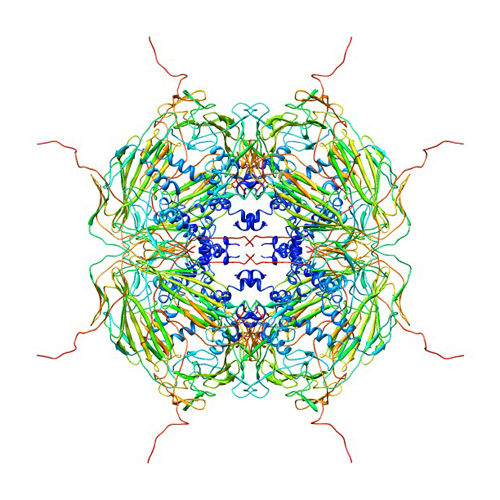
αβ-crystallin is small containing 20kDa that is normally formed into oligomers that are around 600kDa. There are three sections of the αβ-crystallin, the α- crystallin domain which is ninety residues long and made of β sheets folded into a sandwich like formation with β8-β-9β-3(β-2) and β4-β4- β6+7. (green Link) On either side of the ACD are the N and C- terminals. The N- terminal (Green Link) is sixty residues long and the C- terminal twenty five residues long (green link). It has been found it is these terminals that are essential for the proper functioning of the αβ-crystallins. Studies conducted by removing parts of the N and C- terminals shoed as decrease in chaperone activity each with their own role in the proper functioning of the αβ-crystallin. The N-Terminal contains a hydrophobic interactive sequence (41STSLSPFYLRPPSFLRAP58) (green link) which is extremely important in substrate selection. It is the nature of this sequence that recognizes and selects substrates to protect against aggregation based on the amount of protein unfolding. The C- Terminal on the other hand contains a polar interactive sequence (155PERTIPITREE165)( Green Link) which imperative for conserving the solubility of the unfolded proteins. These regions have been proven to be conserved among different species. A study conducted in The Netherlands worked with mice and found the same sequence in hamsters. This is the closest, αβ-crystallin is extremely conservative, this proteins rarely undergoes mutations. Though these proteins are do not change very often, when assembled into oligomers they are extremely polydiverse, this makes it very hard to study this group of proteins. This continuous reconfirmation is said to be a result of the residues on HRI(green link) because of its lack of structure.
The sections of αβ-crystallin that are integral to the performance of the protein the terminals merely explain how it is that these little proteins have the ability to prevent apoptosis or mitosis of cancer cells. Much of the function of these proteins has to do with where and how they bind to other molecules, which occurs at numerous binding sites. Many studies have been carried out to determine the binding sites and it wasn’t until recently that scientists unveiled some information about this process. In a pin array study conducted in 2006 by the Mason Eye Institute, where results indicated that binding sites can be found on the sequences 9-20, 43-48, 113-120, 131-138, 141-148. In addition to effective bonding sites there are parts of this molecule that are essential for their chaperone function. Chaperone proteins help proteins assemble or disassemble. These special chaperone sequences include the mentioned C- Terminal and N-Terminal sites along with 75FSVNLDVK82 (β3) (green link), 131LTITSSLS138 (β8) (green link), 141GVLTVNGP149 (β9) ( green Link). The αβ-crystallin family thrives upon the presence of stress and therefore functions best when exposed to varying levels of pH, temperature, certain metal ions, and ATP. A study was conducted in 2006 to tests the effects of the mentioned stresses. The results showed that chaperone activity increased under high temperatures, acidic conditions, whereas the Ca+ and ATP had no affect on the activity of αβ-crystallin. These conditions mimic the environments that are present when certain disorders occur.