Allosteric modulation of H-Ras GTPase
From Proteopedia
| |||||||||
| 3k8y, resolution 1.30Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Gene: | HRAS, HRAS1 (Homo sapiens) | ||||||||
| Related: | 2rge, 3k9n, 3k9l | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
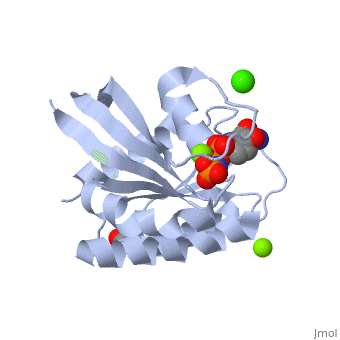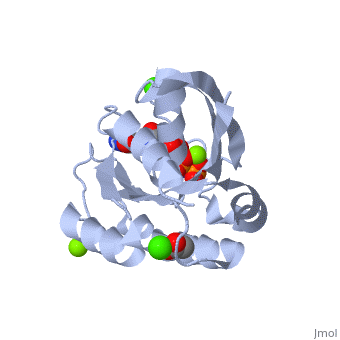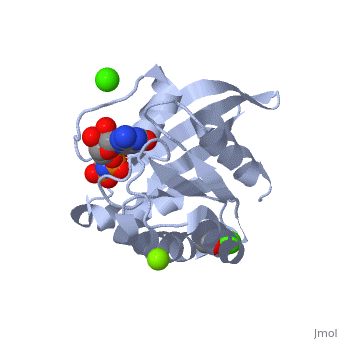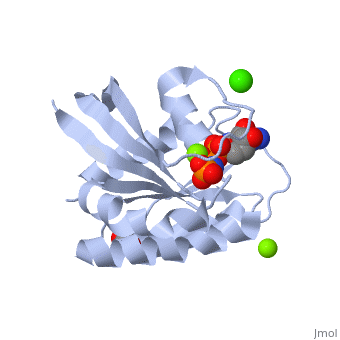
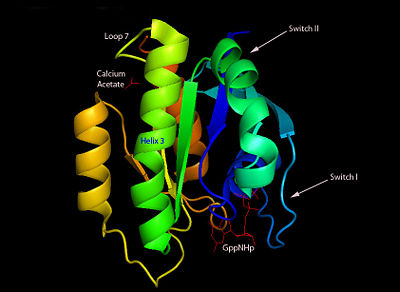
H-Ras is a small monomeric GTPase protein that is found inside of cells. It helps regulate cell division in cells through signal transduction pathways such as the Ras pathway. H-Ras is also known as a molecular switch, it contains two switch regions: Switch I and Switch II. Switch I is a Thymine 35 and switch II is a Glycine 60, both of these switches depend on the phosphate group on a GTP molecule to turn “on” and “off”. In the Ras catalytic cycle, Ras is activated when it attaches to a GTP molecule turning both switch regions “on”. This pathway also involves GTPase activating protein, which hydrolyzes the GTP attached to the Ras causing the switches to turn “off”.
Structure
H-Ras is 166 Amino Acids long with six beta sheets and six helices. One of the helix 2 is a 3/10 helix, unlike the rest of the helices. Also it contains a Calcium Acetate in the allosteric binding site that is located by the Loop 7 and Helix 3. The structure of Ras actually forms slightly different when it is and isn't bound to calcium acetate. The GTP molecule will then attach on the bottom of the protein inbetween the Switch I and Switch II region.Mutations
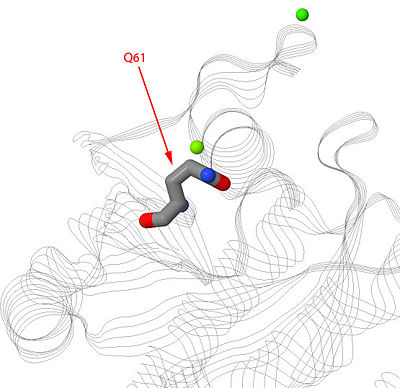
Mutation is Q61 causes shift in amino acids