This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1cfc
From Proteopedia
|
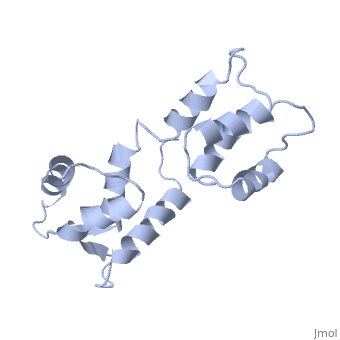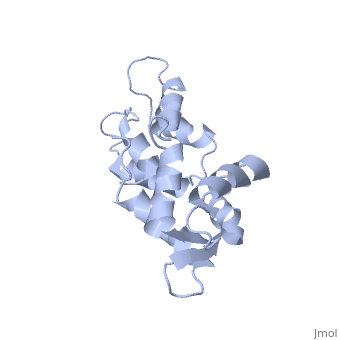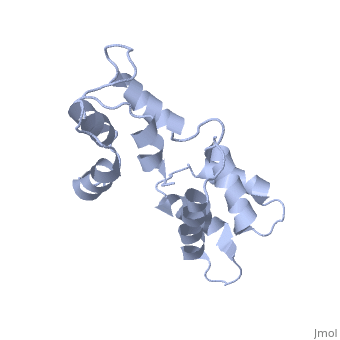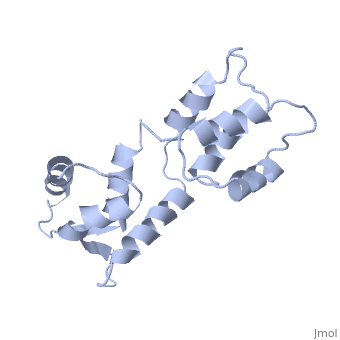
CALCIUM-FREE CALMODULIN
Overview
The three-dimensional structure of calmodulin in the absence of Ca2+ has been determined by three- and four-dimensional heteronuclear NMR experiments, including ROE, isotope-filtering combined with reverse labelling, and measurement of more than 700 three-bond J-couplings. In analogy with the Ca(2+)-ligated state of this protein, it consists of two small globular domains separated by a flexible linker, with no stable, direct contacts between the two domains. In the absence of Ca2+, the four helices in each of the two globular domains form a highly twisted bundle, capped by a short anti-parallel beta-sheet. This arrangement is qualitatively similar to that observed in the crystal structure of the Ca(2+)-free N-terminal domain of troponin C.
About this Structure
1CFC is a Single protein structure of sequence from Xenopus laevis. Full crystallographic information is available from OCA.
Reference
Solution structure of calcium-free calmodulin., Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A, Nat Struct Biol. 1995 Sep;2(9):768-76. PMID:7552748
Page seeded by OCA on Thu Feb 21 12:05:32 2008