Protein secretion is essential for all organisms. Bacteria have evolved a plethora of secretion systems to handle the various proteins that need to be exported from the cytoplasm [1]. Most of these proteins bear an amino terminal extension termed signal peptide and they are called preproteins. The Sec system is uniquitus and essential for cell viability. In bacteria, the Sec system consists of the membrane embedded heterotrimeric complex SecYEG that forms the channel through which preproteins are threaded and the cytoplasmic nanomotor SecA. SecA spends chemical energy to produce mechanical work that somehow pushes and threads preproteins through SecYEG.
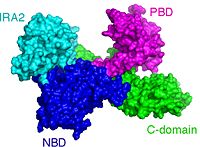
SecA constists of 4 domains; the (NDB); the (PBD); the (IRA2) and the (C-domain). SecAs from different organisms have been visualized [2]. The most prominent difference between the protomers of visualized SecAs is an 80o rotational and translational movement of the around its that connects it with NBD.
So far no functional role has been unequivocally atributed to this rotational motion. It has been suggested that PBD rotates to allow opening of a clamp-like structure and arrest preproteins destined to be secreted. SecA has been visualized in a ; and a Closed conformation.