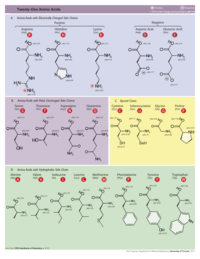
Amino Acids
From Proteopedia
For a general introduction to amino acids, please see Amino Acids in Wikipedia.
Contents |
20 Standard Amino Acids and Mnemonics
Here are the names of the twenty standard amino acids, with their three and one-letter abbreviations. Mnemonic names are intended to help you to remember the one-letter codes, but are not the correct names.
|
Ala A Alanine
|
Gln Q Glutamine |
Leu L Leucine
|
Ser S Serine
|
Click on the image below to see it full size. It shows the 20 amino acids plus one unusual amino acid, selenocysteine.
Unusual Amino Acids
Post-translational modifications of amino acids include phosphorylation and nitrosylation, e.g. to produce nitrotyrosine. However, there are at least two non-standard amino acids that are genetically encoded, discussed below.
Selenocysteine: the 21st amino acid
Rare proteins in all domains of life include selenocysteine (Sec, U). Over a dozen entries in the PDB include selenocysteine, identified as CSE.
Pyrrolysine: the 22nd amino acid
Some proteins in methanogenic archaea include pyrrolysine (Pyl). Methanosarcina barkeri monomethylamine methyltransferase (MtmB, 1nth, 1l2q) was the first identified structure containing this amino acid, and in the crystal structure it is identified as BGX, with the rest of the amnio acid identified as LYS202[1]. Chemically modified forms of pyrrolysine are present in 1tv2, 1tv3, and 1tv4[2]. The PDB also contains several structures for pyrrolysyl-tRNA synthetase.
No 23rd amino acid?
The 21st and 22nd amino acids are specified by the stop codons UGA and UAG respectively, modified with downstream stem-loop structures in the mRNA. Lobanov et al.[3] searched "16 archaeal and 130 bacterial genomes for tRNAs with anticodons corresponding to the three stop signals". Their data suggest that "the occurrence of additional amino acids that are widely distributed and genetically encoded is unlikely."
Structure, Properties, Behaviors in Proteins
Angel Herráez has provided a tutorial introduction to amino acid structure in which each of the 20 amino acids may be visualized in 3D using Jmol.
Please help to expand the list below to include all 20 amino acids.
Glycine
Gly G: Small.
See Glycine in Wikipedia, where the structure is shown. Glycine is the smallest amino acid, since its side-chain is nothing but a single hydrogen. Glycine is frequently found in turns, since the steric clashes of larger amino acids would be problematic. Turns typically occur on the surfaces of proteins, between beta-strands or alpha-helices. It is common to find highly conserved glycines in turns on protein surfaces, since mutation to a bulkier residue would interfere with the fold. Glycine also occurs in beta-strands and alpha-helices, although its frequency in alpha helices is low (second lowest, after proline, p. 125 in [4]). Glycine is commonly found at the C-terminus of alpha helices, and is considered a helix terminator (p. 125 in [4]).
Histidine
His H: Charged: Basic, Aromatic, Bulky
See Histidine in Wikipedia, where the structure is shown. The sidechain of His can be positively charged (protonated), in which case both of the nitrogens in the sidechain imidazole ring have hydrogens, and the charge is delocalized between them. The pKa for protonation is 6.1. This means that, on average at any moment, half of the His sidechains are protonated when the pH is 6.1. At the pH of blood, 7.4 ±0.05, His sidechains are positively charged less than 10% of the time. Therefore, His is not included in the usual list of positively charged amino acids (lysine and arginine).
Phenylalanine
Phe F: Neutral, Aromatic, Bulky
See Phenylalanine in Wikipedia, where the structure is shown. Phe participates in Cation-pi interactions. Phenylketonuria is a genetic disease in which the enzyme that converts phenylalanine to tyrosine is nonfunctional, leading to toxicity from excess phenylalanine, and tyrosine deficiency.
Tryptophan
Trp W: Neutral, Polar, Aromatic, Bulky
See Tryptophan in Wikipedia, where the structure is shown. In trans-membrane proteins, tryptophans often lie at the interface between water and lipid. An example is the potassium channel, e.g. 1bl8. To see their positions, use the Find dialog in FirstGlance in Jmol. Trp participates in Cation-pi interactions.
Tyrosine
Tyr Y: Neutral, Polar, Aromatic, Bulky
See Tyrosine in Wikipedia, where the structure is shown, and Nitrotyrosine. Tyr participates in Cation-pi interactions.
See Also
References
- ↑ Hao B, Gong W, Ferguson TK, James CM, Krzycki JA, Chan MK. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science. 2002 May 24;296(5572):1462-6. PMID:12029132 doi:10.1126/science.1069556
- ↑ Hao B, Zhao G, Kang PT, Soares JA, Ferguson TK, Gallucci J, Krzycki JA, Chan MK. Reactivity and chemical synthesis of L-pyrrolysine- the 22(nd) genetically encoded amino acid. Chem Biol. 2004 Sep;11(9):1317-24. PMID:15380192 doi:10.1016/j.chembiol.2004.07.011
- ↑ Lobanov AV, Kryukov GV, Hatfield DL, Gladyshev VN. Is there a twenty third amino acid in the genetic code? Trends Genet. 2006 Jul;22(7):357-60. Epub 2006 May 19. PMID:16713651 doi:10.1016/j.tig.2006.05.002
- ↑ 4.0 4.1 Kessel, Amit, and Nir Ben-Tal. Introduction to Proteins: Structure, Function, and Motion. CRC Press, 2011. 653 pages.