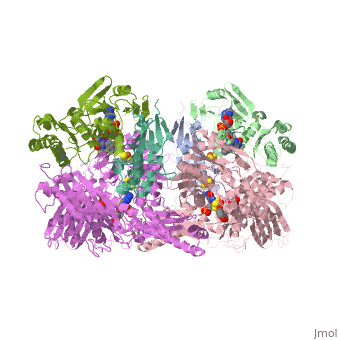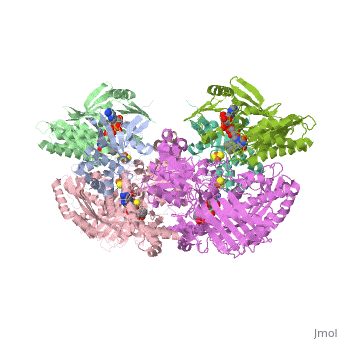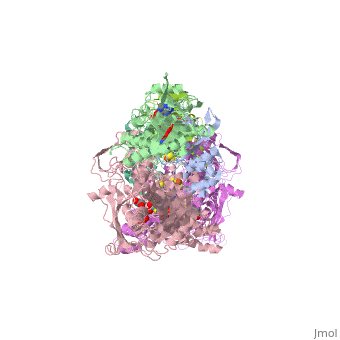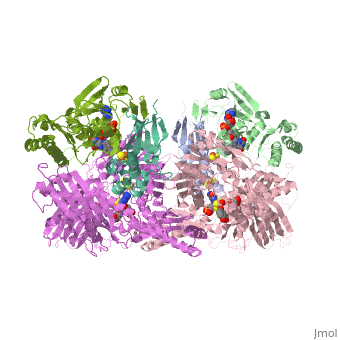
Xanthine oxidoreductase
Xanthine oxidoreductase is actualy considered to exists as two enzymes in one, one being xanthine oxidase and the other existing as xanthine dehydrogenase. When the enzyme was initially purified by scientists two different forms of the enzyme were found, where one uses nicotinamide adenine dinucleotide (NAD) and the other uses oxygen. The two forms were believed to be two different enzymes and were thus given two different names; however, upon further investigation into the amino acid sequence of the enzymes it was determined that the enzymes were actually the same. The two forms of the enzyme can differ in two ways:
1. There are several disulfide bridges within the oxidoreductase enzyme and if these bridges are left intact the enzyme acts as an oxidase, but if these bridges are cleaved the enzyme acts as the dehydrogenase.
2. The oxidoreductase enzyme can be permanently cleaved by proteases so that it always acts in the oxidase form.
Metabolism
Electron Extraction
One side of the xanthine oxidoreductase enzyme consists of an active site that includes a molybdenum atom which binds to a purine substrate and adds a hydroxyl group. During this process electrons are extracted and funneled from the active site through a string of iron-sulfur clusters to the opposing side of the enzyme. The opposing side then transfers the electrons to NAD or oxygen depending on the dehydrogenase or oxidase nature of the enzyme. One of the final steps in the electron transfer funnels electrons to a FAD group. The dehydrogenase form of the enzyme transfers these electrons to NAD, while the oxidase form blocks NAD through a loop of protein that covers the FAD molecule allowing smaller oxygen molecules to accept the electrons.