Introduction
Grb10 is a member of a family of adapter proteins (Grb7 and Grb14) that interacts with tyrosine kinases. [1]
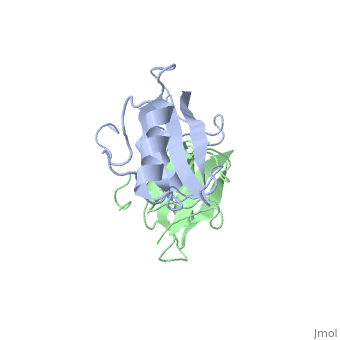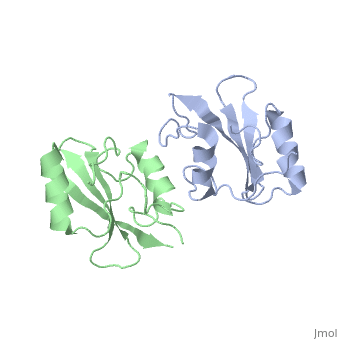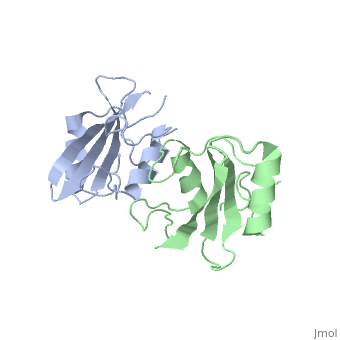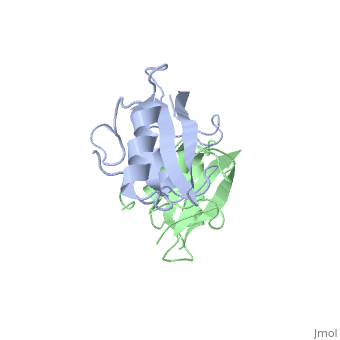
Dimerization of the Grb10 SH2 Domain
The crystal structure of Grb10 SH2 domain was an important step to understanding how this protein interacts with IGF1 receptors, and although the SH2 domain functions as an independent segment, it forms a dimer in physiological environments. The exists due to non-covalent intermolecular forces between Phenylalanine515, Tyrosine516, and Asparganine519 of each independent monomer [1]. The structure of Grb10 SH2 forms similar SH2 domains found in other proteins, which have an on the outsides with anti-parallel .