Introduction
Grb10 is a member of a family of adapter proteins (Grb7 and Grb14) that interacts with tyrosine kinases. [1]
Dimerization of the Grb10 SH2 Domain
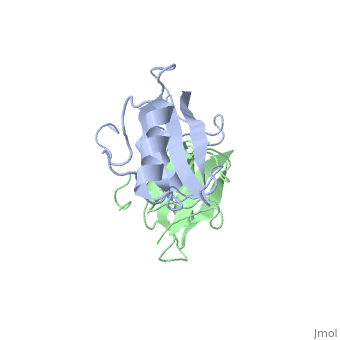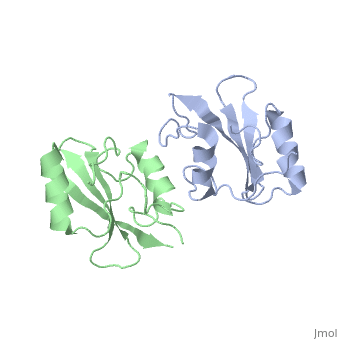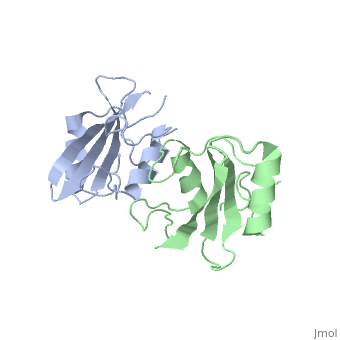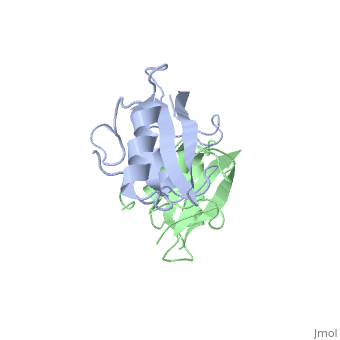
The crystal structure of Grb10 SH2 domain was an important step to understanding how this protein interacts with IGF1 receptors, and although the SH2 domain functions as an independent segment, it forms a dimer in physiological environments. The exists due to the middle hydrophobic Phe515 and uncharged Thr504 (labeled blue) residues packed to the its equivalent counter parter on the other protomer, designated as Phe515' and Thr504' (labeled red); Gln511 (blue) forms a hydrogen bond to the backbone of Asp514' (red) while the side chain of Asn519 forms two hydrogen bonds to the backbone of Lys505'. [1]. The interface ends with Leu518 and Phe-496' via hydrophobic interactions. [1]. The structure of Grb10 SH2 forms similar SH2 domains found in other proteins, which have an on the outsides with anti-parallel .