Sandbox Reserved 647
From Proteopedia
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
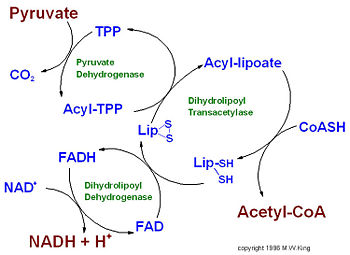
IntroductionPyruvate dehydrogenase (E1) along with two other enzymes make up the pyruvate dehydrogenase multienzyme complex. Dihydrolipoyl transacetylase (E2) and dihydrolipoyl dehydrogenase (E3) are the other two main components of the complex, along with the associated prosthetic groups, Thiamine pyrophosphate (TPP), Lipoamide, and Flavin Adenine Dinucleotide (FAD) respective to each enzyme. These enzymes work together to synthesize acetyl Co-A by the irreversible oxidative decarboxylation of pyruvate before entering the Kreb's cycle. This catalytic reaction also produces NADH and carbon dioxide as by-products. The pyruvate dehydrogenase complex acts to link glycolysis to the citric acid cycle. The complex is localized in the matrix space of the mitochondria in eukaryotes and in the cytosol of bacteria. This complex is responsible for regulating the flow of energy in cells by determining when pyruvate should be converted into acetyl Co-A or neutralized to lactic acid to allow continued glycolysis. ‘[1]’
StructureIn the active site of pyruvate dehydrogenase lies the prosthetic group, TPP, and the tightly bound metalloenzyme, Magnesium. These cofactors are required for catalysis, and the catalytic Mg2+ ion coordinates octahedrally with three protein residues, The structure of pyruvate dehydrogenase (E1) was solved using NMR and X-Ray crystallography, however the entire complex could only be constructed using cryo-EM images.
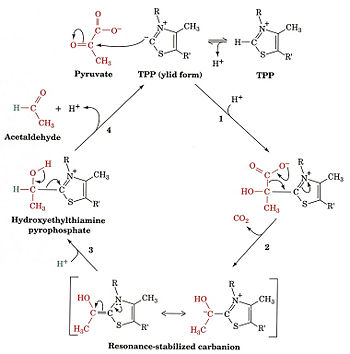
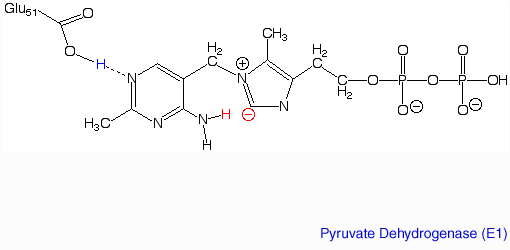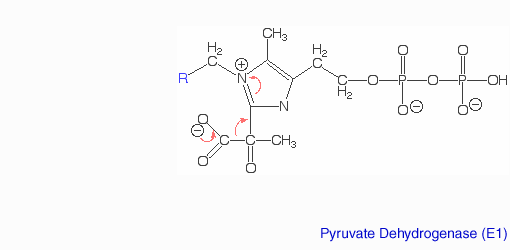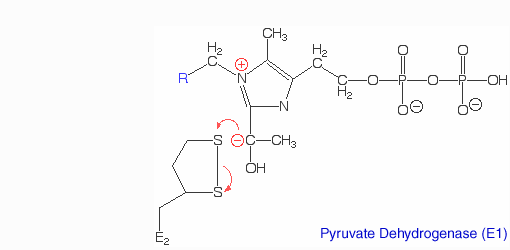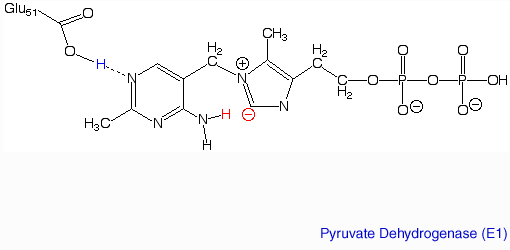
The pyruvate dehydrogenase complex is the largest multienzyme complex known to exist with a molecular weight of about 9 Mega Daltons. The core of this multienzyme complex is composed of 60 (E2) subunits that form the shape of a pentagonal dodecahedron. This shape is composed of 20 vertices, with each vertex being occupied by an (E2) trimer. Each of these subunits has a linker region containing the lipoamide swinging arm that makes contact with the (E1) subunits that surround the inner core. The outer shell of the complex is composed of 60 (E1) subunits. Each enzyme contacts one of the underlying (E2) enzymes. The (E3) enzyme lies in the center of the pentagon formed by the core of the (E2) enzymes and are associated with small binding proteins that are part of the complex. The pyruvate dehydrogenase enzyme itself is a tetramer that consists of two alpha subunits and two beta subunits and is present in 30 copies. Pyruvate dehydrogenase consists of a combination of beta strands and alpha helices. ‘[2]’ Prokaryotes In relation to the eukaryotic dehydrogenase complex, bacteria have a smaller version of the complex where there are only 24 (E2) enzymes that make up the core. The core of the complex is arranged in a cubical shape with 8 vertices with each vertex being occupied by an (E2) trimer. The (E2) subunit of the complex is evolutionarily related to that of the eukaryotic enzyme. However the (E1) subunit found in bacteria is unrelated to that of the eukaryotic (E1) subunit. Bacterial (E1) is a homodimer with a molecular weight of 99,474 Daltons containing and falls in the class of alpha and beta proteins. ‘[3]’ Function and MechanismE1 has two catalytic sites. The α- subunit binds magnesium ion and the thiamine pyrophosphate while the β-subunit binds the pyrimidine fragment of TPP, forming together a catalytic site at the interface of the subunits. A proton readily dissociates from the carbon that is between the nitrogen and sulfur in the thiazole ring of the prosthetic group (TPP) that is associated with the enzyme pyruvate dehydrogenase. The keto carbon of pyruvate reacts with the carbanion of TPP on E1 to yield an additional compound. The electron-pulling positively charged nitrogen of the thiazole ring promotes loss of CO2. What remains is hydroxyethyl-TPP. The hydroxyethyl carbanion on TPP of E1 reacts with the disulfide of lipoamide on E2. The active site for pyruvate dehydrogenase holds TPP through metal ligation to a magnesium ion and by hydrogen bonding to amino acids.‘[4]’‘[5]’
RegulationThe pyruvate dehydrogenase complex is regulated through reversible phosphorylation. The phosphorylation events occur on three serine residues at sites and are located on the pyruvate dehydrogenase (E1) alpha subunit. Once all three of the serine residues are phosphorylated the entire complex is inhibited, thus preventing the synthesis of Acetyl-CoA. The phosphorylation events are catalyzed by the enzyme pyruvate dehydrogenase kinase (PDK). Pyruvate dehydrogenase kinase exists in four known isoforms PDK1-PDK4. These kinases are distributed differently in various tissues. Activation of these kinases are also regulated by different factors. PDK1 is activated when there is a low concentration of oxygen available. PDK2 is activated when there is a high amount of Acetyl-CoA and NADH already present. PDK3 is activated when there is a high abundance of ATP already accessible for use. PDK4 is activated when the cell is suffering from starvation and nutrient deprivation. Dephosphorylation of the serine residues to restore the activity of the pyruvate dehydrogenase complex is catalyzed by the enzyme pyruvate dehydrogenase phosphatase (PDP). This enzyme has two known isoforms PDP1 and PDP2. PDP1 is prevalent in skeletal muscles and PDP2 is prevalent in the liver and adipocytes.
Medical Implications and ApplicationsPyruvate dehydrogenase complex deficiency (PDCD) is one of the most common neurodegenerative disorders associated with abnormal mitochondrial metabolism. The key features of this condition are gray matter degeneration with foci of necrosis and capillary proliferation in the brainstem. This deficiency causes the body to be deprived of energy that results from carbohydrates. The pyruvate dehydrogenase complex is responsible for the production of Acetyl-CoA, which feeds into the citric acid cycle to produce ATP directly and NADH and QH2 indirectly as sources of energy. Thus preventing pyruvate from being converted into Acetyl-CoA, results in abnormal lactate buildup and energy deprivation. The treatment that is available for patients suffering from PDCD is cofactor supplementation with thiamine, and lipoic acid. The cases of this disorder that are responsive to these cofactors respond to supplementation. Evidence shows that high doses of thiamine may be the most effective way to counteract the deficiency. ‘[6]’ References‘
|