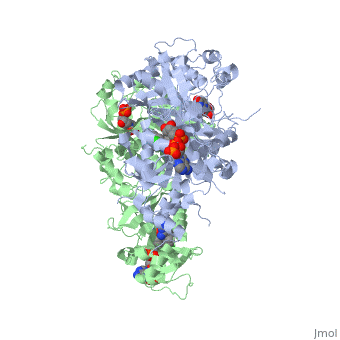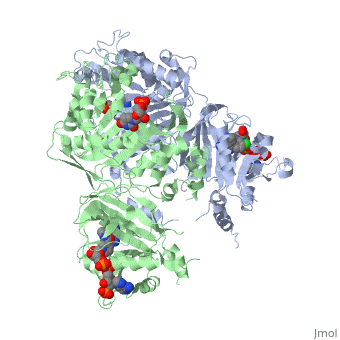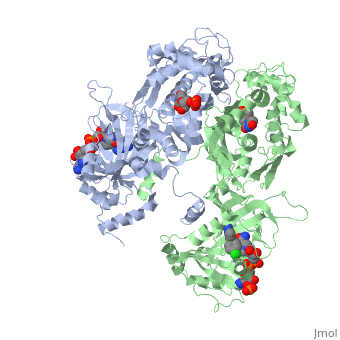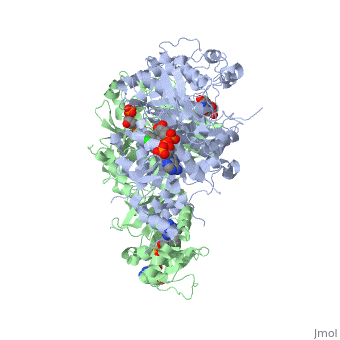
Mutant, Drug Resistant PfDHFR
The
of PfDHFR has four
: N51I, C59R, S108N, and I164LYuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16823-8. Epub 2012 Oct 3. PMID:23035243. doi: 10.1073/pnas.1204556109.</references>. These mutations cause decreased binding affinities of inhibitors, a huge factor being steric clashing. For pyrimethamine and cycloguanil specifically, the N51I mutation greatly effects their ability to bind the enzyme due to the drugs' rigid chlorophenyl substituents. In an effort to mitigate the effect of the mutations, the commonly occurring quadruple mutant must be addressed in the research of new PfDHFR targeting drugs.
A first step in this direction was to experiment with the drug that, with its (2,4,5-trichlorophenoxy)propoxy side chain, addressed the steric clash that was subjected to with a S108N mutation. However further research with this drug was stopped because of its gastrointestinal toxicity in humans and low bioavailbility. Wr99210 was significantly more basic than pyrimethamine, a pyrimidine and slightly acidic substance to match that of the gastrointestinal track, and thus was not readily absorbed in the intestines. Though it was effective at getting deep into the mutant PfDHFR active site, its ineffectiveness as an oral drug would have made it unsuccessful.
Species Specificity