SandboxPKA
From Proteopedia
The c-Abl protein 1 (ABL1), also known as Abelson kinase, is a non-receptor tyrosine kinase that plays a role in many key processes linked to cell growth and survival such as cytoskeleton remodeling in response to extracellular stimuli, cell motility and adhesion, receptor endocytosis, autophagy, DNA damage response and apoptosis. [1] [2] Activity of c-Abl protein is negatively regulated by its SH3 domain, and deletion of the SH3 domain turns ABL1 into an oncogene. In more than 90% cases, chronic myelogeneous leukemia (CML) is caused by a chromosomal abnormality that results in the formation of the Philadelphia chromosome. This chromosome is formed by fusion between Abelson (Abl) tyrosine kinase gene at chromosome 9 and break point cluster (BCR) gene at chromosome 22, resulting in the chimeric oncogene BCR-Abl and a constitutively active BCR-Abl tyrosine kinase. Small molecule inhibitors of BCR-Abl that bind to the kinase domain can be used to treat CML [3]
Contents |
Structure
All of the protein kinases have a similar bilobal fold, and their key structural features have been well studied. Like others, the abelson kinase incorporates a highly conserved bi-lobed structure with an adenosine triphosphate (ATP) binding domain situated in a deep cleft between the N- and C-terminal lobes. Adjacent to this is the centrally located activation loop that incorporates a conserved Asp-Phe-Gly (DFG) sequence and controls catalytic activity by switching between different states in a phosphorylation-dependent manner [4].
| |||||||||||
Bcr-Abl tyrosine-kinase inhibitors
Gleevec
Imatinib mesylate (STI571 or Gleevec), discovered in 1992, is a Bcr-Abl tyrosine kinase inhibitor (Bcr-Abl TKI) and it's the most common first generation drug used for the CML treatment. Imatinib has a high affinity for ABL kinase.
Imatinib achieves BCR-ABL kinase inhibition by binding to the inactive, unphosphorylated conformation of the kinase (DFG-out), thereby reducing the availability of the catalytically active phosphorylated conformation (DFG-in), necessary for ATP binding. This blocks signal transduction, ultimately resulting in inhibition of proliferation and loss of viability and proliferation.[5].
Imatinib is used for treating CML, gastrointestinal stromal tumours and other diseases. By 2011, Gleevec has been FDA approved to treat ten different cancers.
Pharmacokinetics: Imatinib is rapidly absorbed when given by mouth, and is highly bioavailable: 98% of an oral dose reaches the bloodstream. Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system. The main metabolite, N-demethylated piperazine derivative, is also active. The half-lives of imatinib and its main metabolite are 18 and 40 hours, respectively.
Nilotinib
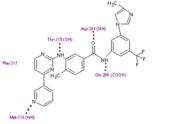
Nilotinib (AMN107) is a second-generation oral TKI. It was designed based on the crystal structure of imatinib to be highly active against a wide range of imatinib-resistant BCR-ABL mutants and is approved for the treatment of newly diagnosed or imatinib-resistant or -intolerant CML. nilotinib was designed to maintain binding to the inactive conformation of the ABL kinase domain, while incorporating alternative binding groups to the N-methylpiperazine moiety and preserving an amide pharmacophore to retain H-bond interactions with Glu286 and Asp381. [6] Substitution of the N-methylpiperazine moiety present in imatinib by a phenyl group bearing trifluoromethyl and imidazole substituents in the nilotinib structure greatly contributes to the potency of nilotinib by reducing the requirement for hydrogen bonding with nilotinib (four H-bond interactions compared with six H-bonds for imatinib). Among patients with imatinib resistant CML, nilotinib has not been associated with the toxic effects commonly seen with imatinib treatment, such as fluid retention, edema, and weight gain [7]
Dasatinib
Dasatinib is also a second-generation TKI. Dasatinib binds kinases of the SCR family (0,2 nM<IC50<1,1nM). It binds both the active and inactiv forms of the Abl kinase with a higher affinity (300 times) than Imatinib. Dasatinib is active against most BCR-ABL mutants with the exception of T315I. [8] Dasatinib is well tollerated but adverse effects can include muscle and joint aches, fatigue, dermatologic complaints such as rash and acne, headaches, and diarrhea [9]
Third generation drugs
New mutations of Bcr-Abl tyrosine kinase are still being discovered and some old go unconquered. To overcome those mutations, new generation drugs are being developed to treat patients with CML.
Bosutinib
On September 4, 2012, the U. S. Food and Drug Administration approved bosutinib tablets (Bosulif, Pfizer, Inc.) for the treatment of chronic, accelerated, or blast phase Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) in adult patients with resistance or intolerance to prior therapy. [10]
is based on a quinoline scaffold and is structurally related to the AstraZeneca quinazoline template. [11]
Reaction
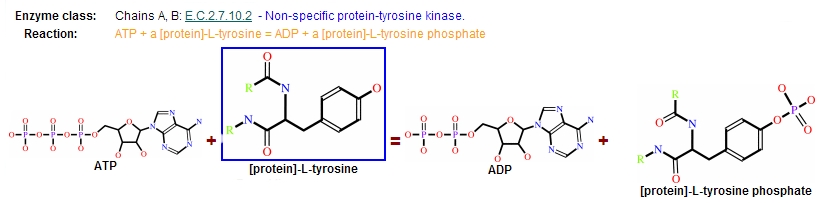
Protein kinases are a group of enzymes that possess a catalytic subunit that transfers the gamma (terminal) phosphate from nucleotide triphosphates (often ATP) to one or more amino acid residues in a protein substrate side chain, resulting in a conformational change affecting side protein function.
The enzymes are classified into two broad groups, characterised with respect to substrate specificity:
- Serine/threonine kinases
- Tyrosine specific kinases: c-Abl is included in this group [12]
Catalytic domain
It is responsible of both, ATP binding as well as protein binding.
| |||||||||||
Resistance
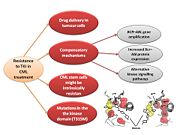
In the majority of cases, resistance is caused by reactivation of Bcr–Abl kinase activity. The F-helix and two hydrophobic spines define the internal architecture of the protein kinase molecule. The R-spine (red surface) and C-spine (yellow surface)are anchored to the F-helix in the middle of the rigid C-lobe. Major elements of the catalytic machinery are also anchored to the F-helix directly or via the spines. In Abl kinase, the gatekeeper is a smaller threonine (Thr 315) that is not an effective stabilizer of the R-spine. Mutation in this residue is the most common mechanism implicated in secondary drug resistance. Usually, gatekeeper threonine is substituted by isoleucine or methionine and avoid Gleevec entrance to ATP-binding domain [13].
In some treated patients, BCR–ABL gene amplification and increased Bcr–Abl protein expression have been observed as a compensatory mechanism for the imatinib antitumour effect. The switch to alternative kinase signalling pathways, which can compensate for the loss of Bcr–Abl activity, has been proposed as another resistance strategy of leukaemic cells. Imatinib might also have different effects on chronic myelogenous leukaemia (CML) tumour cells depending on their differentiation state, and it has been proposed that quiescent CML stem cells might be intrinsically resistant to the drug. Other mechanisms of resistance to imatinib might be related to pharmacokinetic factors of drug delivery. Imatinib can be actively transported out of tumour cells through efflux pump proteins to keep intracellular drug concentrations below inhibitory levels. Extracellular sequestration of imatinib by 1 acid glycoprotein in the plasma has been proposed as a potential mechanism, which would result in reduced availability of the free drug to CML cells [14] .
| |||||||||||
References
- ↑ Yuan ZM, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1437-40. PMID:9037071
- ↑ Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000 Nov 20;19(49):5643-50. PMID:11114745 doi:10.1038/sj.onc.1203878
- ↑ Crystal Structures of the Kinase Domain of c-Abl in Complex with the Small Molecule Inhibitors PD173955 and STI571
- ↑ Schindler T, Bornmann W, Pellicena P, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938 – 42.
- ↑ Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006;2:358 – 64.
- ↑ Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129 – 41.
- ↑ Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosomepositive ALL. N Engl J Med. 2006;354:2542–51.
- ↑ Shah NP, Tran C, Lee FY, et al. (2004) Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305:399–401.
- ↑ Results of Dasatinib Therapy in Patients With Early Chronic-Phase Chronic Myeloid Leukemia Jorge E. Cortes, Dan Jones, Susan O'Brien, Elias Jabbour, Farhad Ravandi, Charles Koller, Gautam Borthakur, Brenda Walker, Weiqiang Zhao, Jianqin Shan and Hagop Kantarjian
- ↑ http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm318203.htm
- ↑ Manley, P.; Cowan-Jacob, S.; Mestan, J. (2005). "Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia". Biochimica et Biophysica Acta 1754 (1–2): 3–13.
- ↑ Leukemia research 34 (10): 1255–1268. doi:10.1016/j.leukres.2010.04.016. PMID 2053738
- ↑ Protein kinases: evolution of dynamic regulatory proteins
- ↑ http://www.nature.com/nrd/journal/v3/n12/full/nrd1579.html