User:Elizabeth R. Haglin/Sandbox 1
From Proteopedia
Biological context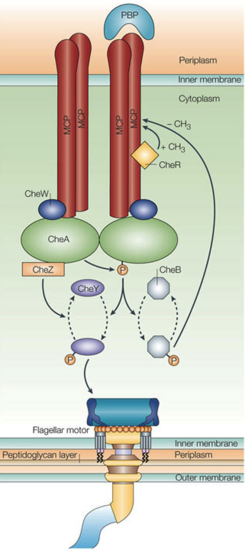 Chemotaxis overview [1] Chemotaxis is a behavior used by most motile flagellated bacteria, like E. coli and T. maritima, to modify their swimming pattern in response to environmental stimuli. Directionality is controlled by the counter-clockwise (CCW) running or clockwise (CW) tumbling motion of the flagellar motor, which in turn is regulated by large arrays of a two-component signal transduction complex responsible for sensing extracellular chemical concentration gradients. Upon binding of a chemical ligand, the MCPs regulate ATP-dependent trans-autophosphorylation activity of the histidine kinase CheA. A repellent binding event leads to increases in phosphorylated CheA (CheA-P) and a subsequent increase in phosphorylation of CheA’s binding partner and CheY. Phosphorylated CheY (CheY-P) has a high affinity for the flagellar motor switch protein FliM and at increased concentrations will change the motor rotation from CCW to CW, leading to tumbling. Likewise, attractant binding inhibits CheA phosphorylation so unphosphorylated CheY dominates, the motor switch CCW motion is unaffected, and the cell maintains smooth swimming. The CheY-P signal is additionally regulated by the phosphatase CheZ. Ultimately, the flux of phosphoryl groups governs the mobility response to external stimuli. StructureThe isolated CheA kinase exists as a homodimer of 71-kDa subunits, each of which catalyzes ATP-dependent trans-phosphorylation of a histidine. A CheA monomer contains 5 domains (named P1-P5 from N- to C-terminus) connected by highly dynamic linkers of various lengths. Each domain has a distinct and important function.
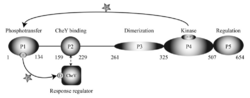
P1P2: Histidine phosphotransfer domainsP3P4P5: Kinase core domainsBinding partnersAdditional 3D structures of CheAReferences</StructureSection> | ||||||||||||