Sandbox Reserved 716
From Proteopedia
|
Crystal structure of P-selectin lectin/EGF domains complexed with SLeX
Contents |
Introduction
Selectins are proteins that are include in a family of cell adhesion receptor involved in the leukocyte extravasation. There are 3 kinds of selectins :
E selectin localized in endothelial cells, L selectin found in leukocytes, and P selectins in platelets and endothelial cells.
In this page we will be focused only on P-Selectin.
When the P-selectin is activated by molecules like histamine or thrombin, it is able to bind myeloid cells and some T cells.
3D structure
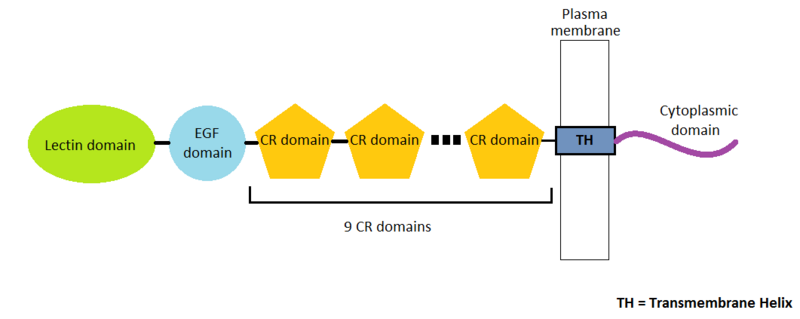
P-Selectin is a protein composed by 162 amino-acids residues in 4 different chains A, B, C and D. There are many domains in this protein. Three types of domains represent the extracellular part of the protein : the EGF domain, the lectin domain and nine consensus repeat (CR) domains. Finally, we find a transmembrane helix and a cytoplasmic domain.
EGF domain seems to be useful for three kind of interactions :
- ligand recognition : The main ligand for P-selectin is P-selectin glycoprotein ligand-1 (PSGL-1)
- cell adhesion : adhesion of the leukocyte to the vessel membrane
- protein-protein interactions
The P-selectin lectin domain binds via an extended site of the domain a ligand : the carbohydrate sialyl LewisX (SLeX) with low affinity.
Different role of the P-selectin
Role in leukocyte extravasation
Leukocyte extravasation is the movement of leukocytes out of the circulatory system at the time of an infection. The inflammation is a natural reaction of defense caused by tissular damages. The recruitement of leukocytes on the inflammation site is a process in four steps. First, the leukocyte is attracted by cytokines, secreted near the site of infection. This is the chemoattraction. Then, this leukocyte begin rolling along the inner surface of the vessel, binding on selectin molecules. The third step is the tight adhesion, the leukocyte immobilizates himself, thanks to the integrin molecules. And finally, he passes through gaps between endothelial cells. By this mecanism, the leukocyte arrives on the site of infection to neutralize the infection agent.
Role in platelets recruitment
Role in cancer
External resources
Protein Data Bank file 1G1R
References
1. http://cro.sagepub.com/content/10/3/337.full.pdf
2. Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000 Oct 27;103(3):467-79. PMID 11081633.
3. Kansas GS, Saunders KB, Ley K, et al (1994). "A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion". J Cell Biol 124 (4): 609–18. PMID 7508943.
4. Phan UT, Waldron TT, Springer TA (2006). "Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force". Nat Immunol 7 (8): 883–9. doi:10.1038/ni1366. PMID 16845394.
Proteopedia Page Contributors and Editors
Delphine Trelat, Cécile Ehrhardt