Group:MUZIC:Myostatin
From Proteopedia

|
Contents |
Introduction
Myostatin which is also known as growth and developmental factor-8(GDF-8) was originally identified in a screen for novel mammalian members of the transforming growth factor-ß (TGF-ß) superfamily of growth and differentiation factors.The phenotype of myostatin knock-out mice suggested that myostatin functions as a negative regulator of muscle growth, and it was on this basis that myostatin was given its name [1]. For these reasons, inhibitors targeting myostatin have been regarded as potential drugs in the treatment of muscle-wasting disorders such as muscular dystrophy [2].
Structure
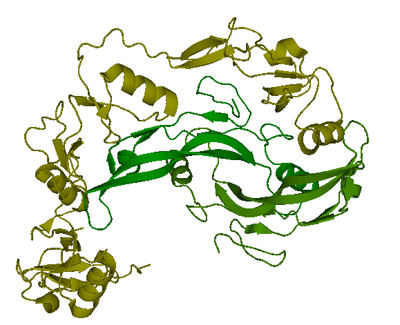
Only one structure of myostatin is currently available in Protein Data Bank. The complex of two follistatin 288 molecules bound to one myostatin dimer was resolved to 2.15 Å using X-ray crystallography and deposited in PDB. (Green:myostatin C-terminal dimer, yellow: follinstatin 288)
Function and Interactions
Myostatin is initially formed as a precursor protein which undergoes two proteolytic processing events in order to generate the biologically active molecule. First the N-terminal signal sequence is removed, a second cleavage generates the C-terminal fragment, which possesses receptor-binding activity and modulates a signal transduction cascade in the target cell [3]. The N-terminal fragment after proteolytic processing has been referred to as the propeptide. One mechanism for activating myostatin latency appears to be proteolytic cleavage of the propeptide [4]. In addition to the regulation of intracellular myostation processing,follistatin has been known to be capable of binding and inhibiting the activity of the myostatin C-terminal dimer. [5].
The interaction of myostatin with titin-cap(T-cap),a Z-disk protein which binds to N-terminal domain of titin,was identified by a yeast two-hybrid system. [6] It is presumed that myostatin has a putative role in the muscle Z-disk regulation.
Pathology
References
- ↑ McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997 May 1;387(6628):83-90. PMID:9139826 doi:10.1038/387083a0
- ↑ Bradley L, Yaworsky PJ, Walsh FS. Myostatin as a therapeutic target for musculoskeletal disease. Cell Mol Life Sci. 2008 Jul;65(14):2119-24. PMID:18425412 doi:10.1007/s00018-008-8077-3
- ↑ McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997 May 1;387(6628):83-90. PMID:9139826 doi:10.1038/387083a0
- ↑ Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15842-6. Epub 2003 Dec 11. PMID:14671324 doi:10.1073/pnas.2534946100
- ↑ Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990 Feb 16;247(4944):836-8. PMID:2106159
- ↑ Nicholas G, Thomas M, Langley B, Somers W, Patel K, Kemp CF, Sharma M, Kambadur R. Titin-cap associates with, and regulates secretion of, Myostatin. J Cell Physiol. 2002 Oct;193(1):120-31. PMID:12209887 doi:10.1002/jcp.10158