Image:Screen shot 2013-05-07 at 2.01.54 PM.png
From Proteopedia
No higher resolution available.
Screen_shot_2013-05-07_at_2.01.54_PM.png (494 × 396 pixel, file size: 41 KB, MIME type: image/png)
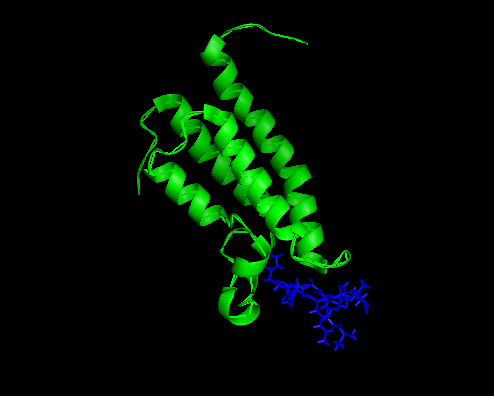
Cartoon showing the solution structure of the bromodomain of human BRPF1 in complex with histone H4K5ac peptide (PDB2RS9). Like all known bromodomains, the BRPF1 bromodamain adopts a conserved structural fold of a left-handed bundle of four helices (αZ, αA, αB, and αC ) with the inter-helical ZA and BC loops having variable length and sequence. This variable regions make a hydrophobic pocket constituting the binding site.
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 19:40, 7 May 2013 | Mulu Lubula (Talk | contribs) | 494×396 | 41 KB | Cartoon showing the solution structure of the bromodomain of human BRPF1 in complex with histone H4K5ac peptide (PDB:2RS9).Like all known bromodomains, the BRPF1 bromodamain adopts a conserved structural fold of a left-handed bundle of four helices (αZ, |
| 19:30, 7 May 2013 | Mulu Lubula (Talk | contribs) | 494×396 | 41 KB | Cartoon showing the solution structure of the bromodomain of human BRPF1 in complex with histone H4K5ac peptide (PDB2RS9). Like all known bromodomains, the BRPF1 bromodamain adopts a conserved structural fold of a left-handed bundle of four helices (αZ, |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
