Sandbox Reserved 770
From Proteopedia
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Phenylalanine Ammonia Lyase
|
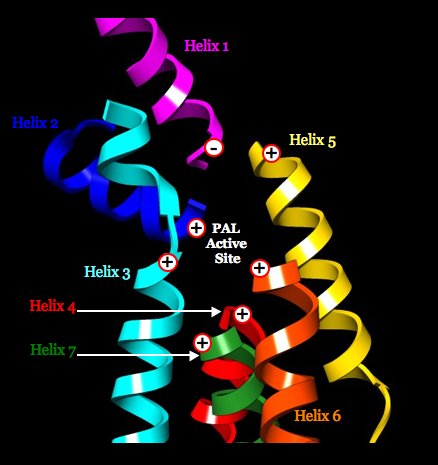
We propose that catalysis (including lowering the pKa of nonacidic C3 of 1-phenylalanine for an E1cb mechanism) is potentially governed by dipole moments of seven alpha helices associated with the PAL active site (six positive poles and one negative pole).
Cofactor MIO resides atop the positive poles of three helices, for increasing its electrophilicity.
The helix dipoles appear fully compatible with a model of phenylalanine docked in the active site of PAL having the first covalent bond formed between the amino group of substrate and the mehtylidene group of MIO: 12 highly conserved residues (near the N termini of helices for enhancing function) are poised to serve roles in substrate recognition, MIO activation, product separation, proton donation, or polarizing electrons from the phenyl ring of substrate for activation of C3; and highly conserved His residue (near the C terminus of the one helix that directs its negative pole toward the active site to increase the residue’s basicity) is positioned to act as a general base, abstracting the pro-S hydrogen from C3 of substrate. A similar mechanism is proposed for HAL, which has a similar disposition of seven alpha helices and similar active-site residues. The helix dipoles appear incompatible with a proposed mechanism that invokes a carbocation intermediate.