Calsequestrin-2 (or CASQ2) is the soluble Ca2+ binding protein in the sarcoplasmic reticulum lumen of the cardiac muscle cells. CASQ2 could be either in a monomeric, homodimeric, or homooligomeric chain form depending on its bounds with Ca2+. Mutations of CASQ2 are involved in cardiac diseases such as Catecholaminergic Polymorphic Ventricular Tachycardia.
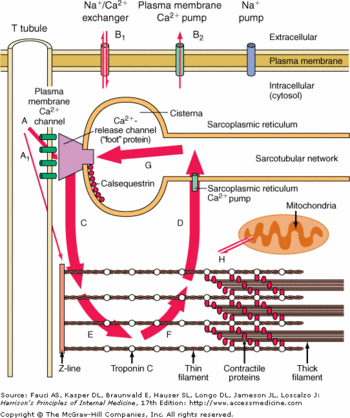
Calsequestrin in the calcium cycle of myocyte contraction
Biological role
The contractions of cardiac myocytes are triggered by the increase of calcium concentration in the cytosol. This phenomenon is highly controlled at several levels. First the calcium is stocked in a cell compartment called the sarcoplasmic reticulum. Then the release of calcium in the cytosol is dependent of the myocytes membrane depolarization. Finally the release of calcium is extremely brief, as soon as the depolarization is over, the calcium is actively pumped in the sarcoplasmic reticulum.
The calsequestrin 2 plays a major role here, because it helps the release of the calcium in the cytosol while the membrane depolarization occurs and traps the calcium inside the lumen of the sarcoplasmic reticulum.
It is also good to notice that a huge release of calcium in the cytosol would be lethal to the cell, since the calcium would precipitate with the free phosphate groups.
Structure
Monomere Structure
Each monomer is divided in : , and the domains. Each of these has a regular structure: a surrounded by .
Usually these domains are involved in redox phenomena, which lead to disulfide bounds creation. Here these domains are inactive but play an important role in the polymerization of CASQ2.
Polymer Structure
Inside the sarcoplasmic reticulum lumen, CASQ2 polymerizes to form , homotetramers and homooligomers.
Calcium Binding
Interaction between CASQ2 and RYR
Regulation of CASQ2
There are and .
can bind the Ca2+ especially the and the .
The Ct domain is highly implicated in the Ca2+ bounds.
The Ca2+ is bound with the interaction of at least 2 acidic amino acids (glutamate or aspartate). These amino acids are in the external part of the protein. When Ca2+ binds to these amino acids there is a structural change which increase the number of alpha helix. Without Ca2+, there are 10-13% of alpha helix but in presence of Ca2+ there 20->35% of alpha helix.
The N-term domain is implicated in front-to-front dimer interactions. While the C-term domain is involved in back-to-back dimer interactions.

