Sandbox Reserved 911
From Proteopedia
| This Sandbox is Reserved from Jan 06, 2014, through Aug 22, 2014 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 911 through Sandbox Reserved 922. |
To get started:
More help: Help:Editing |
|
Contents |
Introduction
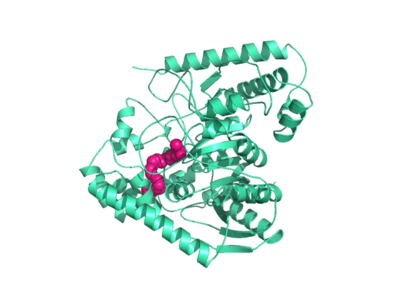
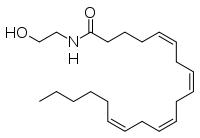
Fatty acid amide hydrolase (FAAH) degrades fatty acid amides to terminate their signaling activity. A serine hydrolase from the Amidase signature superfamily of enzymes (other amidases), FAAH degrades endocannabinoid signaling lipids, molecules associated with pain relief. Because endocannabinoids are lipid molecules, they cannot be compartmentalized in vesicles (the degradation method for other neurotransmitters) and must instead be degraded in the bilayer of the cell membrane. FAAH is an integral membrane protein that degrades FAAs as they enter the membrane bilayer. Current research about FAAH aims to find inhibitors for the enzyme, which would prolong the pain alleviation provided by endocannabinoid molecules.
Hydrolase Information
with
alpha helices 18 and 19 form a membrane binding cap
Catalytic Triad
Applications
FAAH primarily degrades anandamide (AEA), a naturally-occurring lipid that functions in the brain. AEA brings pain relief to the body. Inhibiting FAAH would likely sustain AEA signaling, leading to prolonged pain relief and decreased inflammation.