Binding Pocket
The specificity of the DPP IV in its ability to discern the proline from other amino acids can be seen in the binding pocket, where two glutamates, , fill space, allowing only small residues like proline or alanine to fit. The glutamates form a salt bridge with the N-terminus, positioning the substrate so that only two amino acids can fit into position for hydrolyses. [2] Examples of DPP IV substrates with alanine or proline at their N-terminus are:
| Substrate
| N-terminus
| Function
|
| GLP-1
| His-Ala-Glu-
| Increases insulin secretion; decreases glucagon secretion
|
| GIP
| His-Ala-Asp-
| Increases insulin secretion; decreases glucagon secretion
|
| NPY
| Tyr-Pro-Ser-
| Vasoconstrictor; growth of fat tissue
|
| CXCL12
| Lys-Pro-Val
| Chemotaxis of lymphocytes; angiogenesis
|
[2]
Active Site
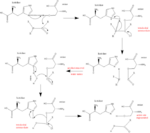
These substrates, along with many others, are cleaved by DPP IV at its active site containing a catalytic triad composed of . This Serine-Histadine-Asparatate motif, best known in the enzyme chymotrypsin, uses acid-base chemistry to facilitate the binding, cleaving, and release of the given substrate. The mechanism of the reaction is as follows:
- Substrate binds to enzyme and carbonyl carbon is positioned by active site.
- Histadine, via hydrogen bond with asparatate, becomes more electronegative and therefore readily accepts the hydrogen from the -OH group on serine, making it nucleophilic.
- The nucleophilic serine attacks the carbonyl carbon, generating a tetrahedral intermediate (as seen in the arrow pushing mechanism).
- The peptide bond is cleaved and the electrons from it move to attack the hydrogen on the histadine. This half of the substrate now dissociates, leaving the other half still bound to serine.
- Histadine then deprotonates a water molecule, forming a nucleophilic hydroxide group that attacks the serine-bound carbonyl carbon.
- As the electrons move from the oxygen back down to reform the double bond, serine dissociates as a leaving group and the other half of the substrate dissociates from the enzyme.
- The negative oxygen on serine readily accepts the hydrogen from histadine and in doing so regenerates the active site of the enzyme.