User:Alexander Rudecki/Sandbox 1
From Proteopedia
|
Contents |
Introduction
Drosophila melanogaster glutaminyl cyclase (DromeQC but also known as CG10487 CG32412 or Dmel\CG32412) is a globular protein part of the α/β-hydrolase superfamily. DromeQC is an aminoacyltransferase (EC 2.3.2.5) that acts on N-terminal glutamine or glutamate residues, producing a stable cap resistant to protease degradation. The human orthologue of DromeQC (hQC) has been implicated in stabilizing amyloid Aβ peptides involved in neurodegenerative disorders such as Alzheimers[1]. It has been shown that DromeQC has a similar overall fold to hQC, as well as a conserved active site[2]. Thus DromeQC is an attractive candidate for transgenic models and mechanistic studies.
Gene->Protein
DromeQC is encoded by chromosome 3L, locus 64F4-64F5 in the D. melanogaster genome[3]. It is transcribed into a 1622 nucleotide transcript, containing 5' (36 nucleotides) and 3' (83 nucleotides) untranslated regions [3]. The translated protein contains 340 residues corresponding to a M=38,028 Da[4]. It contains a 27 residue signal sequence, suggesting its involvement in the secretory pathway [1].
Structure
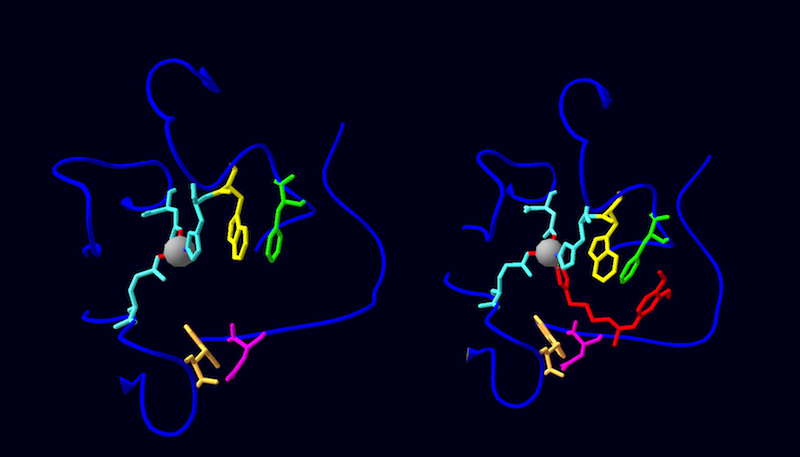
Drosophila melanogaster glutaminyl cylcase (QC) is made up of 2 identical, independent monomers that come together to form an asymmetric homodimer. The subunits are connected via 4 hydrogen bonds (Chain 1→Chain 2: ARG35 NH2→GLU64 OE2, ARG43 NH2→ASN71 O, ARG43 NH2→PHE75 O, ARG43 NH1→PHE75 O) and surface complementarity. The subunits exhibit a globular α/β-hydrolase fold, characterized by a central twisted β-sheet motif consisting of 5 parallel strands (β1 and β3-β6) and an antiparallel β2 strand. This β-center is flanked by 9 surrounding α-helices; 2 fill the concave face (α5, α7), 7 fill the convex face (α1- α5, α8, α9) with one helix at the edge (α6) of each monomer. Within each subunit is 1 cysteine bond (C113→C136) linking the β3 strand to the α3 helix. Each monomer chelates a catalytic zinc ion via D131 OD2, E171 OE2, and H297 NE2, and is glycosylated (with up to 7 carbohydrate moieties) at the N42 position.
References
- ↑ 1.0 1.1 Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat Med. 2008 Oct;14(10):1106-11. doi: 10.1038/nm.1872. Epub 2008 Sep 28. PMID:18836460 doi:http://dx.doi.org/10.1038/nm.1872
- ↑ Koch B, Kolenko P, Buchholz M, Ruiz Carrillo D, Parthier C, Wermann M, Rahfeld JU, Reuter G, Schilling S, Stubbs MT, Demuth HU. Crystal Structures of Glutaminyl Cyclases from Drosophila melanogaster Reveal Active Site Conservation between Insect and Mammalian QCs. Biochemistry. 2012 Aug 16. PMID:22897232 doi:10.1021/bi300687g
- ↑ 3.0 3.1 DromeQC Gene Card. NCBI. [1]
- ↑ DromeQC. UniProt. [2]