Introduction
is a small glycoprotein hydrolase found in the lysosome that breaks the thioester bond between cysteine amino acids and [1]. It is a homodimer that is composed primarily of alpha helices and beta sheets with a hydrophobic groove that allows the palmitic acid[1] to bind, exposing the thioester bond to the catalytic triad. PPT-1 was first found as an enzyme that removed palmitate from GTPase HRas, but over time it has been discovered that is able to remove palmitate from other proteins as well [2]. When PPT-1 is not functioning properly, the lipid modified proteins can build up in the cells and cause infantile neuronal ceroid lipofuscinosis[2][3]

Surface view of PPT-1 showing the hydrophobic groove and palmitate
Catalytic Triad
PPT-1's structure creates an external hydrophobic groove that binds the palmitate acid in between carbon 4 and 5. The acid binds in a gauche conformations creating a kink in the acid chain. This bending could suggest that PPT-1 was originally designed to react with unsaturated fatty acid with cis-double bonds. The catalytic is composed of Serine 115, Aspartate 233, and Histidine 289. The Serine is "deprotonated" by the Histidine and attacks the carbonyl carbon of the palmitic acid. The negative charge is pushed onto the oxygen and is possible stabilized by a water molecule. The tetrahedral collapses and kicks the palmatic acid off of the cysteine residue[2].
Inhibitors of PPT-1
is a lysosomal enzyme which had a serine lipase consensus sequence; a key characteristic of lysosomal enzymes. Despite having a serine lipase consensus sequence, PPT-1, is not deactivated by phenylmethylsulfonyl fluoride (PMSF), a common serine-modifying reagent. (HDSF) is a serine-modifying reagent that is able to inhibit the actions of PPT1 . Unlike other inhibitors, leading away from the active site of PPT-1. PMSF is unable to fit into this small narrow groove due to steric constraints that relate to the unique structure of the substrate-binding site of PPT1. The sulphur of HDSF will via a sulponylation reaction and thus will inhibit the actions of PPT-1.
Disease Associated With PPT-1
Infantile Neuronal Ceroid Lipofuscinosis (INCL) is a recessively inherited disease that is associated with a decrease in PPT-1 activity due to mutations of the PPT-1 enzyme. Onset of symptoms which include retinal blindness, ataxia, seizures, and cortical atrophy of the brain, begin 1-2 years after birth. Death typically occurs between the ages of 8-11. Several mutations in the 1p32 chromosome have been identified to cause INCL.
Mutations Associated with INCL
Juvenile NCL (JNCL) and Late-infantile NCL (LINCL)are less severe forms of INCL in which onset of symptoms occur much later in life, between the ages of 30-40. The mutations associated with JNCL and LINCL occur away from the active site of PPT-1 resulting in a higher activity of the PPT-1 enzyme. A common mutation associated with JNCL and LINCL involves the mutation of Thr-75.
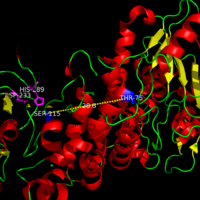
Common Mutation associated with JNCL and LINCL involve mutations Far Away From Active Site
(Bellizzi J. Widom J. Kemp. Lu J. Das K. Hofmann L. Jon Clardy J. 2000. The crystal structure of palmitoyl protein thioesterase1 and the molecular basis of infantile neuronal ceroid lipofuscinosis. Biochemistry. 97: 4573-4578)
Unfortunately not much is known on how to treat INCL. However, in both JNCL and LINCL activity of PPT-1 has only a 2% activity rate compared to normal PPT-1 activity. This suggests that a small increase in activity of PPT-1 may aid in delaying the symptoms associated with INCL. One way in which to increase the activity of PPT-1 is to use protein chaperons that help refold PPT-1 in the endoplasmic reticulm. Although this is not a cure for INCL, by increasing the activity of PPT-1 the life expectancy for individuals with INCL can be greatly increased.
(Dawson G. Schroeder C. Dawson P. 2010. Palmitoyl:protein thioesterase (PPT1) inhibitors can act as pharmacological chaperones in infantile Batten disease. Biochem and Biophys Research Comm. 395. 66-69.)



