[1]
[2]
[3]
Updated on 22-December-2014
Function
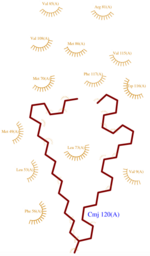
ASP1 is composed of (, residues 8–25; helix 2(scene), residues 27–36; helix 3(scene), residues 42–56; helix 4(scene), residues 66–74 ; helix 5(scene), residues 75–77 ; helix 6(scene),residues 78–90 ;helix 7(scene), residues 96–112)
Helix 1 has a break in the hydrogen-bonding pattern of its structure, forming tight substitute hydrogen bonds with water molecules. Indeed, it results in a kink in helix 1 (at residue Ala 14)(scene) induced by a disruption in the helical conformation, due to hydrogen bonds with water molecules.
The C terminal(scene) domain of this molecule presents a characteristic PBP-GOP domain. While this protein is composed of 144 residues the domain PBP begin at 25 residue. ASP1 binds its ligand at low pH and releases it at neutral pH.
Components implicated in the structure rigidity:
ASP1 presents three disulfides bridges which are greatly enhancing its structure’s rigidity by linking four of the helixes together (scene).
The first disulfide bridge (scene) is established between H1 and H3 through Cysteins 20 and 51. An other disulfide bridge (scene) links H3 and H6 through Cys 47 and 98, and the third and last bridge (scene)connects H5 and H6 thanks to Cys 89 and Cys 107.
Furthermore, non covalent bonds also play an important role.
Indeed, at pH 5.5, Asp 66 and Leu 58 establish an hydrogene bond which is able to lock a key component structure such as H4. (scene)
With their hydrogene bond Val 118 and Ile 119 stabilize alpha helix 2 and C terminal’s position. (scene)
Cavity
The dynamic structure of the protein is responsible of the ligand’s binding by adjustement of position. The structure looses its flexibility when CMJ binds. The successful delivery of the effector to the receptor relies on this property. The ligand accepting entry of the cavity is formed by H2, H4 and H5 (scene). However, the inside of the cavity is formed by the loop between helixes H3 and H4, and the region from H4 to H5(scene). The cavity is prone to accept such ligand because of its specific composition. Indeed, cavity components are mainly hydrophobic and aromatic (scene) and are localized in the same faces of the helix.Thus, it implies that this residues are regularly distant in the primary structure.
Structure
Related Forms
Autres versions de la protéine à pH variable
- 3fe8 The same protein in complex with a serendipitous ligand soaked at pH 4.0
- 3fe9 The same protein in complex with a serendipitous ligand soaked at pH 7.0
- 3cdn The same protein in apo form soaked at pH 4.0
- 2h8v The same protein in apo form at pH 5.5
- 3cz2 The same protein in apo form at pH 7.0
- 3bfa The same protein in complex with the QMP at pH 5.5
- 3bfb The same protein in complex with the 9-ODA at pH 5.5
- 3bfh The same protein in complex with the HDOA at pH 5.5
- 3bjh The same protein in complex with the nBBSA at pH 5.5
- 3cyz The same protein in complex with the 9-ODA at pH 7.0
- 3cz0 The same protein in complex with the QMP at pH 7.0
- 3cz1 The same protein in complex with the nBBSA at pH 7.0
- 3cab The same protein in complex with the nBBSA soaked at pH 7.0
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.