Biological role
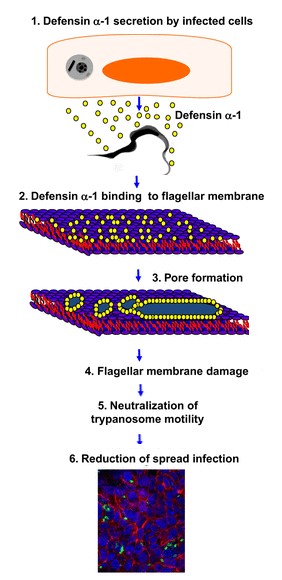
DEF are known to play a role in the in the initiation of innate immune responses to some microbial pathogens. For example the alpha defensin 1 have a role against the bacteria "Trypanosoma cruzi" [3]."Trypanosoma cruzy" or "Cruzy" debilitats Chagas disease, which affects millions of people and products significant morbidity and mortality. The defensine alpha 1 are secreted by the HCT116 cells (which are Paneth cells), when they are infect by "Cruzy". They reduces the infection making damage of the flagella structure. This damage inhibit parasite motility and reduce cellular infection. This reaction is introduce in the following drawing.
 Mevalonate Pathway. Note the early stage at which the statins interfere in the pathway |
Structure
The alpha defensin peptides are secreted inactive. The α-defensins are cationic antimicrobial peptides that are synthesized in vivo as inactive precursors. Activation requires proteolytic excision of their anionic N-terminal inhibitory pro peptide. The pro peptide also specifically interacts with and inhibits the antimicrobial activity of the alpha-defensin intermolecularly.[4]
The active mature α-defensin peptides consist of 29–35 amino acid residues with a molecular mass of 3–5 kDa.
The primary structure shows highly conserved residues, which are indispensable for the structural stability of the peptides. Among them are six invariant cysteine residues, necessary for the typical α-defensin intramolecular disulphide-bond connectivity (Cys1–Cys6, Cys2–Cys4 and Cys3–Cys5). We can observe this bridge on the following figure. There are two charged amino acid residues, Arg5, and Glu13, forming a conserved salt bridge, and Gly17, which constitutes a signature structural motif which is essential for correct folding.
The tertiary structure is a triple-stranded β-sheet with a β-hairpin that contains cationic amino acid residues.
[5]."
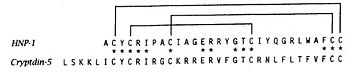 Defensin-Alpha-1 cysteine connectivities. [6]." |
References
- ↑ http://www.ncbi.nlm.nih.gov/gene/1667
- ↑ https://books.google.fr/books Histologie et biologie cellulaire: Une introduction à l'anatomie pathologique Abraham L. Kierszenbaum 2002
- ↑ http://iai.asm.org/content/81/11/4139.full/ref
- ↑ www.ncbi.nlm.nih.gov/pmc/articles/PMC2754386/ )
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2049026/
- ↑ Stephen H Wile, William C Wimley and Michael E Selsted. Structure, function, and membrane integration of defensins. Current Opinion in Structural Biology 1995. University of California, Irvine, USA
Proteopedia page contributors and editors


